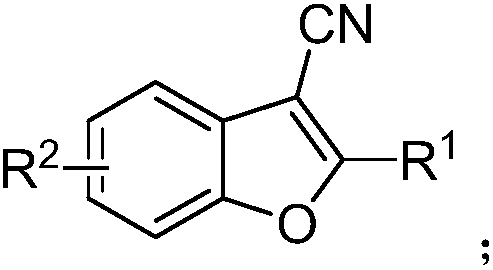
A kind of synthetic method of 2-phenyl-3-cyanobenzofuran compound
A synthesis method and compound technology, which are applied in the field of organic compound cyanation synthesis, can solve the problems of waste of metallic copper, low selectivity and the like, and achieve the effects of reducing processes, readily available reaction raw materials and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0021] Example
Embodiment 1
[0023] Add 5-methyl salicylaldehyde (0.5mmol), Cu(OAc) to the reaction flask at room temperature (20-35℃) 2 (0.1mmol), benzylacetonitrile (0.75mmol), sodium methoxide (2mmol) and DMSO (2ml), then stir and heat to 100°C to react until the 5-methylsalicylic aldehyde reaction is complete. After the reaction, the reaction solution was cooled to room temperature, then added to 20ml of water, and extracted with dichloromethane three times, using 10ml of dichloromethane each time, separated by silica gel chromatography, and distilled under reduced pressure. The yield was 74%. The identification result was: White solid, mp 112–113°C. 1 H NMR(400MHz, CDCl 3 ): δ8.16(d,J=7.6Hz,2H), 7.57–7.46(m,4H), 7.43(d,J=8.4Hz,1H), 7.20(d,J=8.4Hz,1H), 2.47 (s,3H). 13 C NMR(100MHz, CDCl 3 ): δ161.6, 151.7, 134.5, 131.0, 129.1, 127.9, 127.7, 127.3, 126.4, 119.62, 114.5, 111.2, 87.7, 21.32. HRMS: Theoretical calculation value C 16 H 11 NO[M + ],233.0841; Test data: 233.0845.
Embodiment 2
[0025] Add 5-methyl salicylaldehyde (0.5mmol), Cu(OAc) to the reaction flask at room temperature (20-35℃) 2 (0.1mmol), p-toluene acetonitrile (0.75mmol), sodium methoxide (2mmol) and DMSO (2ml), then stir and heat to 100°C to react until the 5-methylsalicylic aldehyde reaction is complete. After the reaction, the reaction solution was cooled to room temperature, and then added to 20ml of water, extracted with dichloromethane three times, using 10ml of dichloromethane each time, separated by silica gel chromatographic column, and distilled under reduced pressure. The yield was 56%. The identification result was: White solid, mp 136–137°C. 1 H NMR(400MHz, CDCl 3 ): δ8.06(d,J=8.4Hz,2H),7.48–7.45(m,1H),7.42(d,J=8.8Hz,1H),7.33(d,J=8.0Hz,2H),7.19 (dd,J=8.4,1.2Hz,1H),2.48(s,3H),2.43(s,3H). 13 C NMR(100MHz, CDCl 3 ): δ162.0, 151.6, 141.6, 134.4, 129.8, 127.4, 126.4, 125.2, 119.5, 114.7, 111.1, 87.0, 21.6, 21.3. HRMS: theoretical calculation value C 17 H 13 NO[M + ],247.0997; Test data: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com