Preparation method of lithium difluoro(oxalato)borate
A technology of difluorooxalate lithium borate and lithium oxalate, which is applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problems of using many types of solvents and complex reactions, etc., and achieve Overcoming the effects of many reaction steps, simple preparation method and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
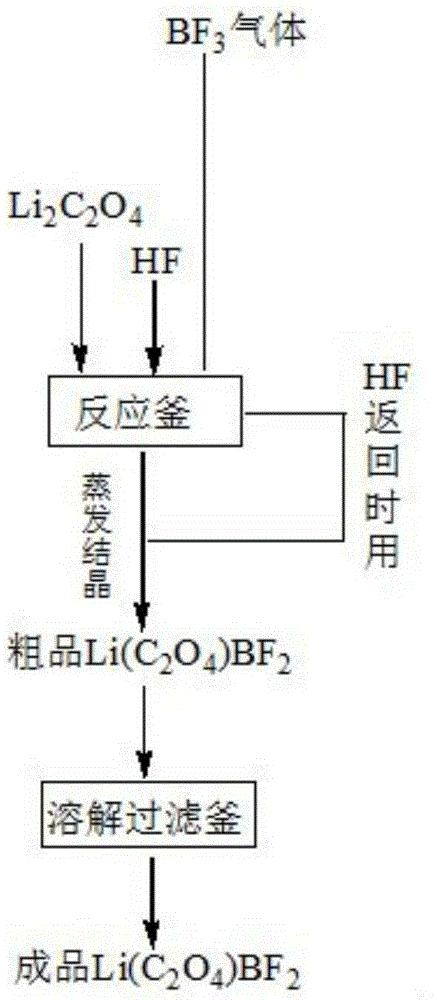
[0017] Example 1: At room temperature, add 101.9g of lithium oxalate and 1300g of HF into a fluorine-lined reaction vessel with a jacket, and stir until the lithium oxalate is completely dissolved in HF. then BF 3 143.02g of gas was slowly passed into the reactor, and the gas export rate was controlled at 2L / Min. During the process, attention was paid to the temperature change at all times, and the temperature was controlled at about 30°C. After the gas was introduced, the stirring was continued for 4 hours. After filtration, 300.12 g of the product was obtained by evaporation and crystallization, and 254.13 g of the crude product were obtained after rapid drying (drying under vacuum for 1 hour, then heating up to 80° C., and drying under vacuum for 3 hours). Finally, the resulting thick product is added to 300gDMC, dissolved and filtered, dried (cold N 2 The temperature was raised to 50°C, and hot N was continuously introduced 2 After three hours, the N 2 After heating up ...
Embodiment 2
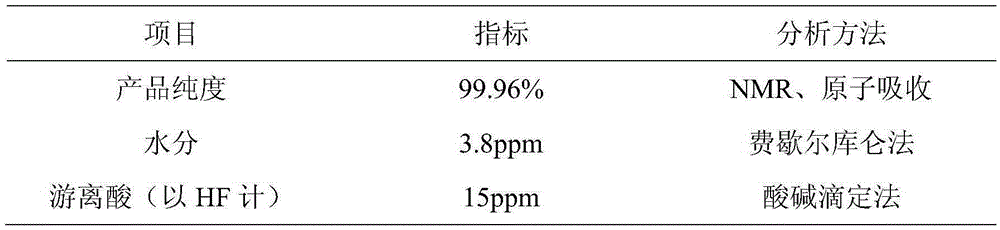
[0020] Example 2: At room temperature, 101.9 g of lithium oxalate and 1300 g of HF were added to reaction vessel 1 with a jacket, and stirred until the lithium oxalate was completely dissolved in HF. then BF 3 143.12g of gas was slowly passed into the reactor, and the gas export rate was controlled at 2L / Min. During the process, attention was paid to temperature changes at all times, and the temperature was controlled below 30°C. After the gas was introduced, the stirring was continued for 4 hours. After filtration, 298.34 g of crude product was obtained by evaporation and crystallization, and 250.12 g of crude product was obtained after rapid drying. Finally, add 300 g of diethyl ether to the obtained crude product, dissolve, filter, and dry to obtain a product quality of 189.27 g, wherein the theoretical yield is 193.79 g, and the product yield reaches 97.66%. The product purity of the obtained product is 99.93%, the water content is 4.8ppm, and the acid content is 20ppm. ...
Embodiment 3
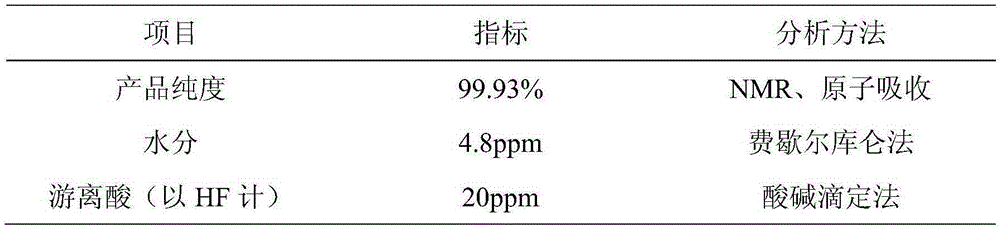
[0023] Example 3: At room temperature, 101.9 g of lithium oxalate and 1300 g of HF were added to reaction vessel 1 with a jacket, and stirred until the lithium oxalate was completely dissolved in HF. then BF 3 144.28g of gas was slowly passed into the reactor, and the gas export rate was controlled at 2L / Min. During the process, attention was paid to the temperature change at all times, and the temperature was controlled at about 40°C. After the gas was introduced, the stirring was continued for 8 hours. After filtration, 301.21 g of the product was obtained by evaporation and crystallization, and 252.27 g of the product were obtained after rapid drying. Finally, add 400g of ethanol to the obtained product, dissolve, filter and dry to obtain a product quality of 187.23g, wherein the theoretical yield is 193.79g, and the product yield reaches 97.61%. The product purity of the obtained product is 99.87%, the water content is 5.2 ppm, and the acid content is 23 ppm.
[0024] Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com