Immobilized type ionic liquid catalytic reaction rectification device
A catalytic reaction and ionic liquid technology, applied in fractionation, organic chemistry, chemical instruments and methods, etc., to achieve the effects of good catalytic activity, easy separation and easy collection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
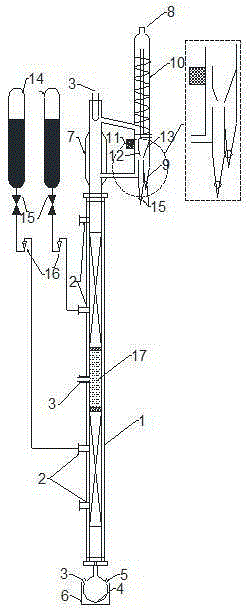
[0030] Such as figure 1 The reactive distillation process shown. Before the experiment started, a certain amount of imidazole-p-toluenesulfonate ionic liquid ([Hmim]TsO / silicagel) immobilized on silica gel was filled in the catalyst fixed layer, and glass spiral packing φ4 was integrally filled in the packing layer. After checking the circuit and switching the valve of the pipe fitting, start the experiment. First open the cooling water of the tower top condenser, from the feed port 5 of the tower kettle, add 300ml of butanol and acetic acid mixture with a molar ratio of 1.2:1, and add 10ml of cyclohexane as a water-carrying agent, and feed through the external heating jacket 6. The tower kettle is heated until it boils so as to wet the tower body packing. Properly adjust the heating voltage to maintain full reflux in the tower, and continue feeding after the whole system is stable. Open the raw material feed valve 15 respectively, and adjust the feed ratio (mole) of butano...
Embodiment 2
[0033] Such as figure 1 The reactive distillation process shown. Before the experiment started, a certain amount of NaY molecular sieve-immobilized imidazole-p-toluenesulfonic acid ionic liquid ([Hmim]TsO / NaY) was first filled in the catalyst fixed layer, and glass spiral packing φ4 was integrally filled in the packing layer. After checking the circuit and switching the valve of the pipe fitting, start the experiment. First open the cooling water of the tower top condenser, from the feed port 5 of the tower kettle, add 300ml of butanol and acetic acid mixture with a molar ratio of 1.2:1, and add 10ml of cyclohexane as a water-carrying agent, and feed through the external heating jacket 6. The tower kettle is heated until it boils so as to wet the tower body packing. Properly adjust the heating voltage to maintain full reflux in the tower, and continue feeding after the whole system is stable. Open the raw material feed valve 15 respectively, and adjust the feed ratio (mole)...
Embodiment 3
[0036] Such as figure 1 The reactive distillation process shown. Before the experiment started, a certain amount of SBA-15 immobilized imidazole-p-toluenesulfonic acid ionic liquid ([Hmim]TsO / SBA-15) was filled in the catalyst fixed layer, and glass spiral packing φ4 was integrally filled in the packing layer. After checking the circuit and switching the valve of the pipe fitting, start the experiment. First open the cooling water of the tower top condenser, from the feed port 5 of the tower kettle, add 300ml of butanol and acetic acid mixture with a molar ratio of 1.2:1, and add 10ml of cyclohexane as a water-carrying agent, and feed through the external heating jacket 6. The tower kettle is heated until it boils so as to wet the tower body packing. Properly adjust the heating voltage to maintain full reflux in the tower, and continue feeding after the whole system is stable. Open the raw material feed valve 15 respectively, through the rotameter 16, adjust the feed ratio ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com