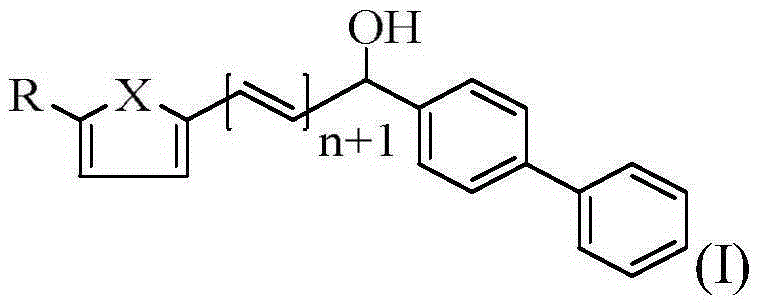
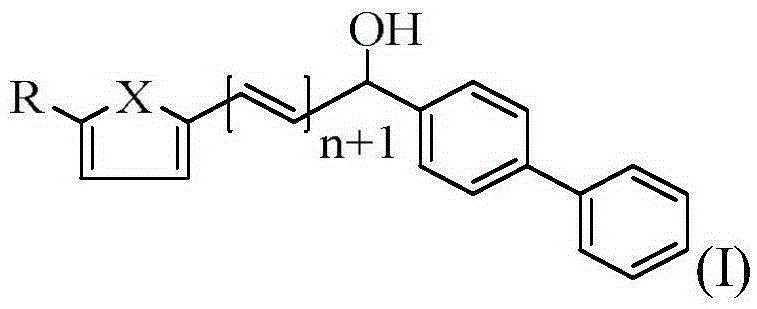
Biphenylmethanol compound as well as preparation method and application thereof
A technology of biphenylmethanol and compounds, which is applied in the field of pesticides, can solve the problems of no biphenylmethanol compounds, etc., and achieve the effects of simple structure, good control effect and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] compound (C 19 h 16 o 2 ) preparation
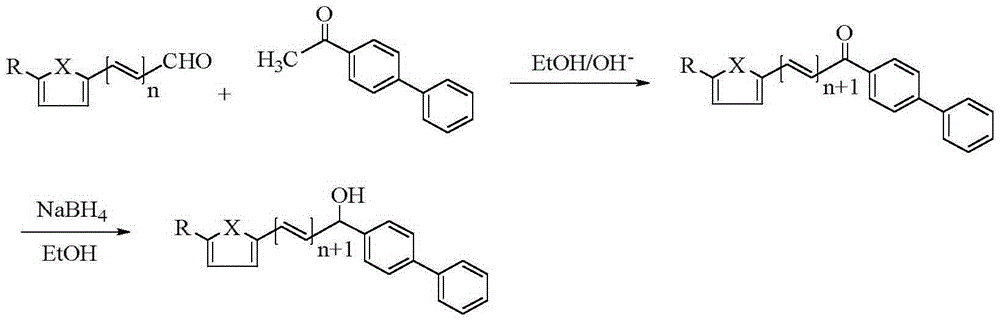
[0033] (1) Synthesis of intermediate 1-(4-biphenyl)-3-(2-furyl)-2-propen-1-one
[0034] Add 0.01mol of 4-phenylacetophenone and 10mL of absolute ethanol into a 50mL three-necked flask, and then add 5mL of 10% NaOH solution into it. Under stirring in an ice bath, slowly drop the mixture of 0.01mol 2-furfuraldehyde and 10mL absolute ethanol into a three-necked flask with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate (TLC ) to check that the reaction is complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-biphenyl)-3-(2-furyl)-2-propen-1-one.
[0035] (2) Synthesis of the target compound
[0036] Add 0.005mol of self-made intermediate and 30mL of absolute ethanol into a 50mL thr...
Embodiment 2
[0039] compound (C 20 h 18 o 2 ) preparation
[0040] (1) Synthesis of intermediate 1-(4-biphenyl)-3-(5-methyl-2-furyl)-2-propen-1-one
[0041] Add 0.01mol of 4-phenylacetophenone and 10mL of absolute ethanol into a 50mL three-necked flask, and then add 5mL of 10% NaOH solution into it. While stirring in an ice bath, slowly drop the mixture of 0.01mol 5-methyl-2-furfuraldehyde and 10mL of absolute ethanol into a three-necked flask with a constant pressure dropping funnel, react at 0-5°C, and use a thin A silica gel plate (TLC) was used to check whether the reaction was complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-biphenyl)-3-(5-methyl-2-furyl)-2-propen-1-one.
[0042] (2) Synthesis of the target compound
[0043] Add 0.005mol of self-made intermediate and 30...
Embodiment 3
[0046] compound (C 19 h 15 OSBr) preparation
[0047] (1) Synthesis of intermediate 1-(4-biphenyl)-3-(5-bromo-2-thienyl)-2-propen-1-one
[0048] Add 0.01mol of 4-phenylacetophenone and 10mL of absolute ethanol into a 50mL three-necked flask, and then add 5mL of 10% NaOH solution into it. While stirring in an ice bath, slowly drop the mixture of 0.01mol 5-bromo-2-thiophenecarbaldehyde and 10mL of absolute ethanol into a three-necked flask with a constant pressure dropping funnel, react at 0-5°C, and use a thin layer Silica gel plates (TLC) were used to check the completion of the reaction. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-biphenyl)-3-(5-bromo-2-thienyl)-2-propen-1-one.
[0049] (2) Synthesis of the target compound
[0050] Add 0.005mol of self-made intermedi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com