A kind of organic charge transport material and preparation method thereof
A charge transport, organic technology, applied in organic chemistry, silicon organic compounds, chemical instruments and methods, etc., can solve the problem of no contribution to electroluminescence, and achieve the effects of easy preparation, improved charge transport performance, and simple purification process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
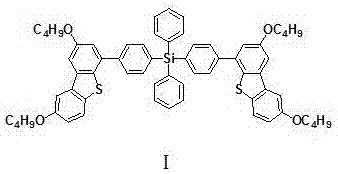
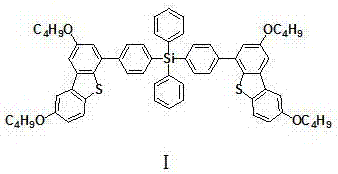
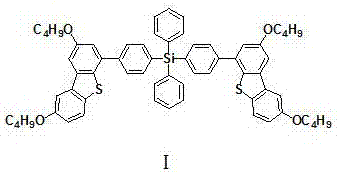
[0028] Embodiment 1, an organic charge transport material, which is based on derivatives of tetraphenyl silicon and butoxydibenzothiophene, the structure is shown in formula I:
[0029]
[0030] Name: Bis{4-[4-(2,8-dibutoxy-2',8'-dibutoxy)(dibenzo[b,d]thienyl)phenyl]}diphenylsilane .
[0031] The preparation method of above-mentioned derivative is as follows:
[0032] .
[0033] The preparation of intermediate II: under the protection of argon, add 74.2g of bis(4-bromophenyl)-phenylsilane and 50ml of tetrahydrofuran to the three-necked flask successively, cool to -78°C, add 225mL of n-butyllithium dropwise, After the dropwise addition was completed, stir at -78°C for 1 hour, then slowly add 124.28 g of tributyl borate dropwise, after the dropwise addition, keep warm and react for 1 hour, the temperature will rise automatically, and the reaction will last overnight. Add 400ml of water, 400ml of concentrated hydrochloric acid, and 550ml of petroleum ether into the reacti...
Embodiment 2
[0036] Embodiment 2, an organic charge transport material, which is based on derivatives of tetraphenyl silicon and butoxydibenzothiophene, the structure is shown in formula I:
[0037]
[0038] Name: Bis{4-[4-(2,8-dibutoxy-2',8'-dibutoxy)(dibenzo[b,d]thienyl)phenyl]}diphenylsilane .
[0039] The preparation method of above-mentioned derivative is as follows:
[0040] .
[0041] The preparation of intermediate II: under the protection of argon, add 37.1g of bis(4-bromophenyl)-phenylsilane and 30ml of tetrahydrofuran to the three-necked flask successively, cool to -78°C, and add 112.5mL of n-butyllithium dropwise After the dropwise addition was completed, stir at -78°C for 1 hour, then slowly add 71.14 g of tributyl borate dropwise, after the dropwise addition, keep warm and react for 1 hour, the temperature will rise automatically, and the reaction will last overnight. Add 200ml of water, 200ml of concentrated hydrochloric acid, and 300ml of petroleum ether into the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com