Electrolyte for lithium secondary battery and lithium secondary battery containing the same
A secondary battery and electrolyte technology, applied in the field of lithium secondary battery electrolyte and lithium secondary battery containing it, can solve the problems of high volatility and high flammability, and achieve improved battery expansion, increased discharge capacity, excellent Effect of high temperature storage characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
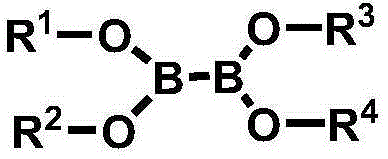
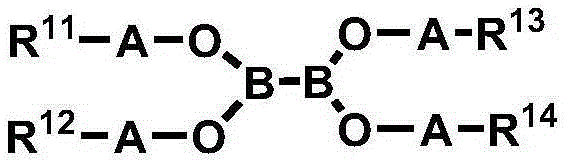
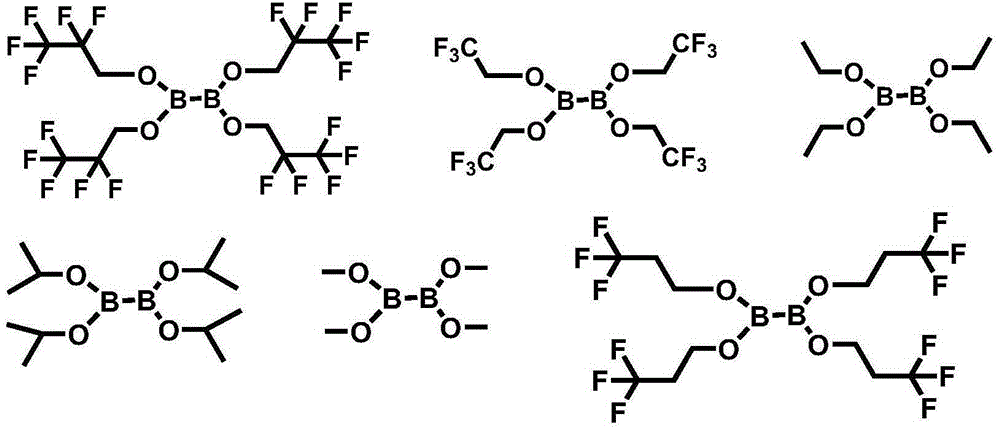
[0114] [Example 1] Tetrakis (2,2,3,3,3-pentafluoropropyl) diboronic acid ester (tetrakis (2,2,3,3,3-pentafluoropropyl) hypodiborate, hereinafter, also known as 'PEA65 ')Synthesis
[0115]
[0116] In a 100ml round bottom flask, 6.00g of 2,2,3,3,3-pentafluoro-1-propanol (2,2,3,3,3-pentafluoro-1-propanol, 40mmol) and 3.96 g of tetrakis(dimethylamino)diboron (tetrakis(dimethylamino)diboron, 20mmol) was dissolved in 30ml of heptane, and then the temperature was raised to 50°C under a nitrogen atmosphere. After reacting at 50° C. for 24 hours, the temperature was lowered to normal temperature. After filtering the crystals generated at normal temperature, the filtrate was vacuum-dried to obtain 8.0 g of the title compound.
[0117] 1 H-NMR (400MHz, CDCl 3 )δ: 4.18(t, J=20.9Hz, 8H)
Embodiment 2-7 and comparative example 1-2
[0119] Dissolve LiPF in a mixed solution of ethylene carbonate (EC): ethylmethyl carbonate (EMC) at a volume ratio of 3:7 6 , to obtain a 1.0M solution, and use this solution as the basic electrolyte (1MLiPF 6 , EC / EMC=3:7), and further put in the components described in the following Table 1 to prepare the electrolyte solution.
[0120] A method for preparing a battery using the non-aqueous electrolyte solution is as follows.
[0121] As the positive active material, LiNiCoMnO 2 and LiMn 2 o 4 Mixed at a weight ratio of 1:1, mixed with polyvinylidene fluoride (PVdF) as a binder and carbon as a conductive agent at a weight ratio of 92:4:4 and dispersed in N-methyl-2 -pyrrolidone, thereby preparing positive electrode slurry. This slurry was coated on an aluminum foil having a thickness of 20 μm, dried and rolled to prepare a positive electrode. Mix artificial graphite as the negative electrode active material, styrene-butadiene rubber as the binder, and carboxymethyl cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com