Meisuoshuli dispersible tablet and preparation method thereof
A technology of mesosulide and dispersible tablets, which is applied in the direction of non-active ingredient medical preparations, pharmaceutical formulas, active ingredients of amides, etc. It can solve the problems of inconvenient taking three times, affecting the overall curative effect, and prone to missed doses, etc. Achieve high oral bioavailability, good medication compliance, and improve dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
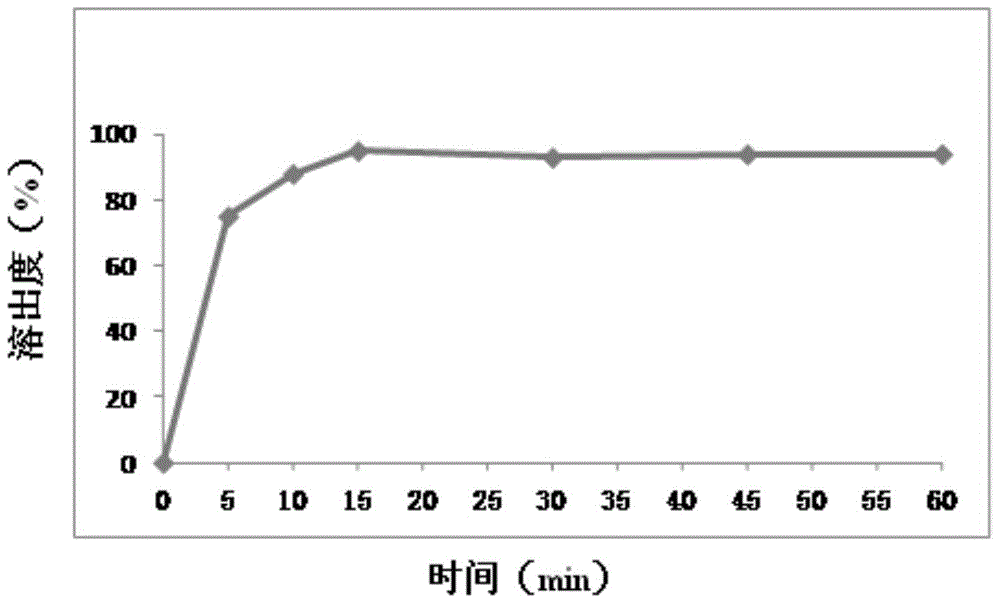
Embodiment 1
[0063] prescription:
[0064] 25 parts by weight of mesosulide, 5 parts by weight of aspartame, 60 parts by weight of microcrystalline cellulose, 5 parts by weight of hydroxypropylmethylcellulose, 2 parts by weight of sodium carboxymethyl starch, 0.5 parts by weight of magnesium stearate
[0065] Preparation:
[0066] (1) The above prescription amount of mesosulide was dissolved in 25ml of acetone to obtain liquid A, and the prescription amount of microcrystalline cellulose and hydroxypropylmethylcellulose were dissolved in 100ml of purified water to obtain liquid B;
[0067] (2) Slowly add liquid A to liquid B while stirring to obtain a mixed mesosulide suspension;
[0068] (3) Homogenize the suspension solution with a homogenizer, then spray dry, and collect the powder;
[0069] (4) the magnesium stearate, aspartame, carboxymethyl starch sodium of powder and prescription quantity are mixed uniformly, get mixed powder, get the sample of mixed powder, and carry out assay, af...
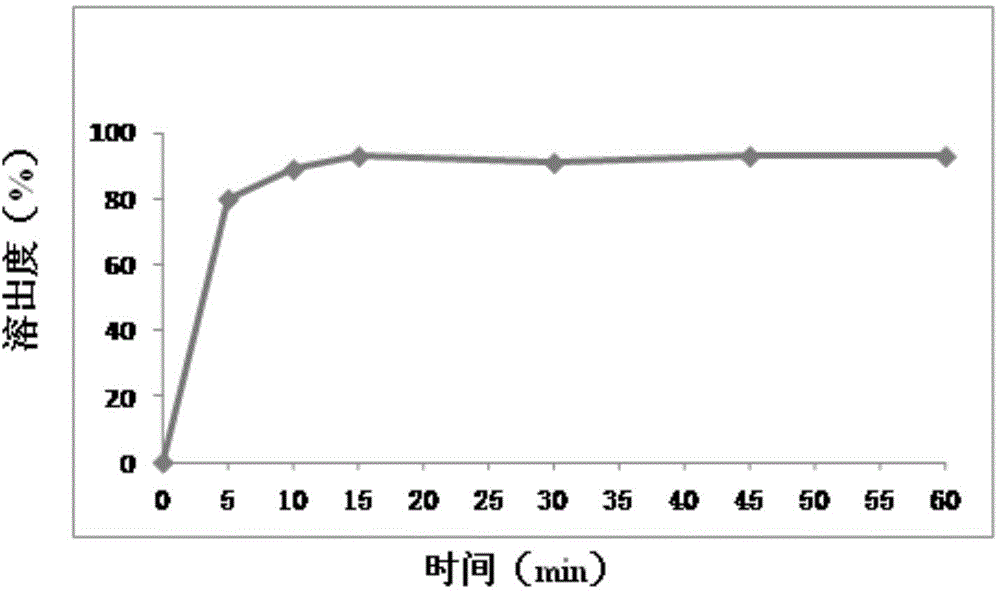
Embodiment 2
[0074] prescription:
[0075] 25 parts by weight of mesosulide, 10 parts by weight of sucrose, 30 parts by weight of lactose, 50 parts by weight of microcrystalline cellulose, 5 parts by weight of povidone, 3 parts by weight of crospovidone, and 0.5 parts by weight of micronized silica gel
[0076] Preparation:
[0077] (1) Dissolve the above prescription amount of mesosulide in 25ml of ethanol to obtain liquid A, and dissolve the prescribed amount of lactose, microcrystalline cellulose and povidone in 100ml of purified water to obtain liquid B;
[0078] (2) Slowly add liquid A to liquid B while stirring to obtain a mixed mesosulide suspension;
[0079] (3) Homogenize the suspension solution with a homogenizer, then spray dry, and collect the powder;
[0080] (4) Mix the powder with the prescribed amount of micropowder silica gel, sucrose, and crospovidone evenly to obtain a mixed powder, take a sample of the mixed powder, and perform content determination, and perform table...
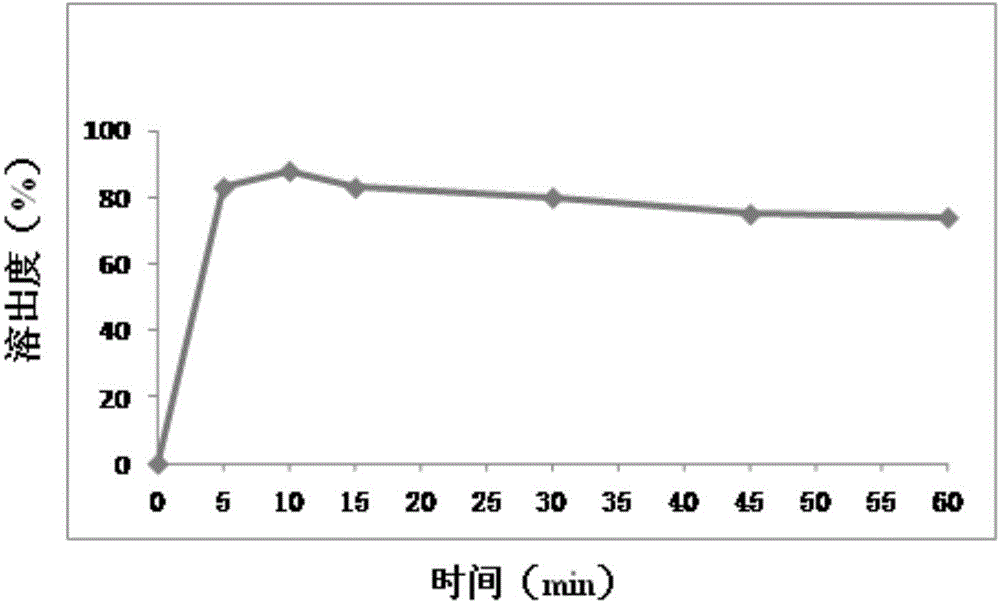
Embodiment 3
[0083] prescription:
[0084] 50 parts by weight of mesosulide, 10 parts by weight of aspartame, 100 parts by weight of microcrystalline cellulose, 10 parts by weight of hydroxypropylmethylcellulose, 5 parts by weight of sodium carboxymethyl starch, 1 part by weight of magnesium stearate
[0085] Preparation:
[0086] (1) Dissolve the above prescription amount of mesosulide in 50ml of ethanol to obtain liquid A, and dissolve the prescription amount of microcrystalline cellulose and hypromellose in 120ml of purified water to obtain liquid B;
[0087] (2) Slowly add liquid A to liquid B while stirring to obtain a mixed mesosulide suspension;
[0088] (3) Homogenize the suspension solution with a homogenizer, then spray dry, and collect the powder;
[0089] (4) the magnesium stearate, aspartame, carboxymethyl starch sodium of powder and prescription quantity are mixed uniformly, get mixed powder, get the sample of mixed powder, and carry out assay, after content is qualified, c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com