Synthesis method of 2,4,6-trimethyl-m-phenylenediamine
A technology of trimethyl m-phenylenediamine and trimethyl, applied in the field of synthesis 2, can solve the problems of high production cost, many reaction steps, and high hydrogenation reduction pressure, and achieves reduction in raw material cost, stable product quality, and high reaction steps. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
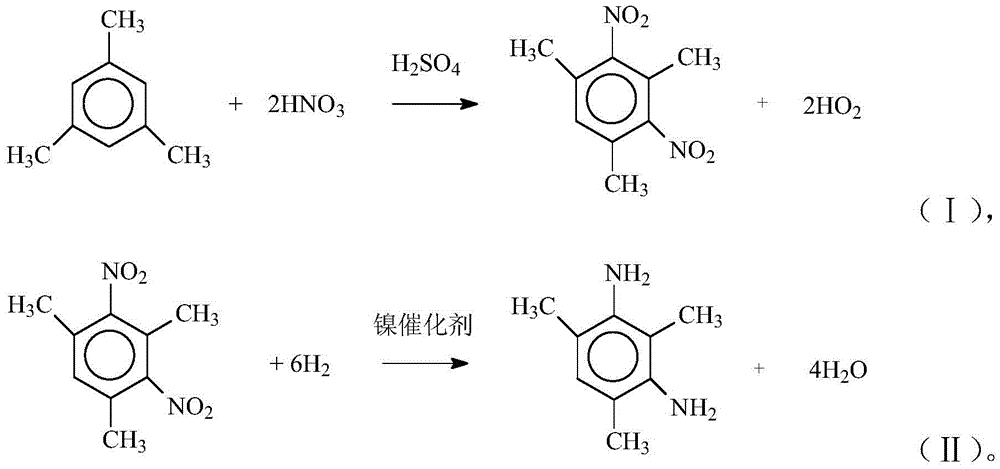
[0017] Add 114kg of mesitylene in 1000L nitration kettle with coil cooling, open and stir and add 700kg of mixed acid dropwise (mixed acid is H 2 SO 4 and HNO 3 of mixed acids, H 2 SO 4 and HNO 3 The weight ratio is 80.4:19.6), the reaction temperature is controlled at 55°C, and the drop is maintained for 30 minutes, the temperature is raised to 95°C, maintained for 30 minutes, the stratification is allowed to stand, and the waste acid is applied to the next batch of nitration reaction. Add 500 kg of water and 2 kg of caustic soda to the organic layer, stir for 1 hour and let it stand for stratification, then add 500 kg of water, and wash with water to obtain 2,4,6-trimethyl-m-dinitrobenzene.
[0018] Add the above-mentioned 2,4,6-trimethyl-m-dinitrobenzene to a 1000L liter autoclave, then add 10kg of Ni catalyst, 320kg of methanol, and replace with nitrogen and hydrogen. Start stirring and heat up to 60°C, start hydrogenation, control hydrogenation pressure 1-3MPa, tempe...
Embodiment 2
[0020] Add 114kg of mesitylene in 1000L nitration kettle with coil cooling, open and stir and add 700kg of mixed acid dropwise (mixed acid is H 2 SO 4 and HNO 3 of mixed acids, H 2 SO 4 and HNO 3 The weight ratio is 75:25), the reaction temperature is controlled at 20°C, the dripping is completed and maintained for 35 minutes, the temperature is raised to 90°C, maintained for 35 minutes, the stratification is allowed to stand, and the waste acid is applied to the next batch of nitration reaction. Add 500 kg of water and 2 kg of caustic soda to the organic layer, stir for 1 hour and let it stand for stratification, then add 500 kg of water, and wash with water to obtain 2,4,6-trimethyl-m-dinitrobenzene.
[0021] Add the above-mentioned 2,4,6-trimethyl-m-dinitrobenzene to a 1000L liter autoclave, then add 10kg of Ni catalyst, 320kg of methanol, and replace with nitrogen and hydrogen. Start stirring and heat up to 65°C, start hydrogenation, control hydrogenation pressure 4MP...
Embodiment 3
[0023] Add 114kg of mesitylene in 1000L nitration kettle with coil cooling, open and stir and add 700kg of mixed acid dropwise (mixed acid is H 2 SO 4 and HNO 3 of mixed acids, H 2 SO 4 and HNO 3 The weight ratio is 87:13), the reaction temperature is controlled at 100°C, the dripping is completed and maintained for 30 minutes, the temperature is raised to 90°C, maintained for 30 minutes, the stratification is allowed to stand, and the waste acid is applied to the next batch of nitration reaction. Add 500 kg of water and 2 kg of caustic soda to the organic layer, stir for 1 hour and let it stand for stratification, then add 500 kg of water, and wash with water to obtain 2,4,6-trimethyl-m-dinitrobenzene.
[0024] Add the above-mentioned 2,4,6-trimethyl-m-dinitrobenzene to a 1000L liter autoclave, then add 10kg of Ni catalyst, 320kg of ethanol, and replace with nitrogen and hydrogen. Start stirring and heat up to 65°C, start hydrogenation, control hydrogenation pressure 2MP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com