Clean technology for continuously synthesizing 2,6-dichloro-p-nitroaniline
A technology for the synthesis of p-nitroaniline, applied in the preparation of organic compounds, preparation of amino compounds, organic chemistry, etc., to achieve significant economic and social benefits, reduce the amount of hydrochloric acid, and achieve effective treatment and comprehensive utilization of resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Continuous synthesis:
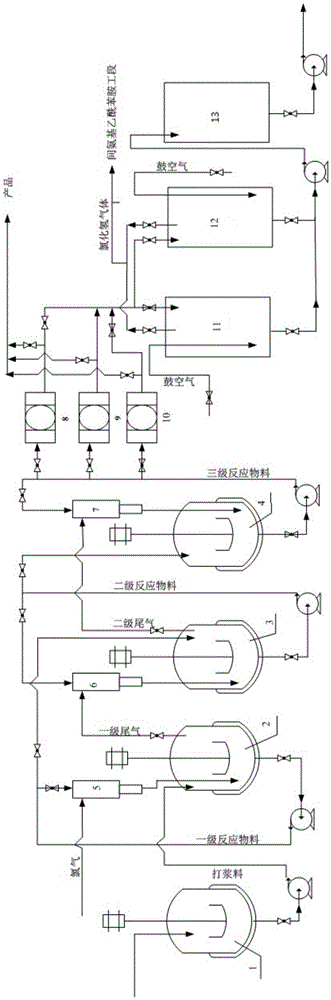
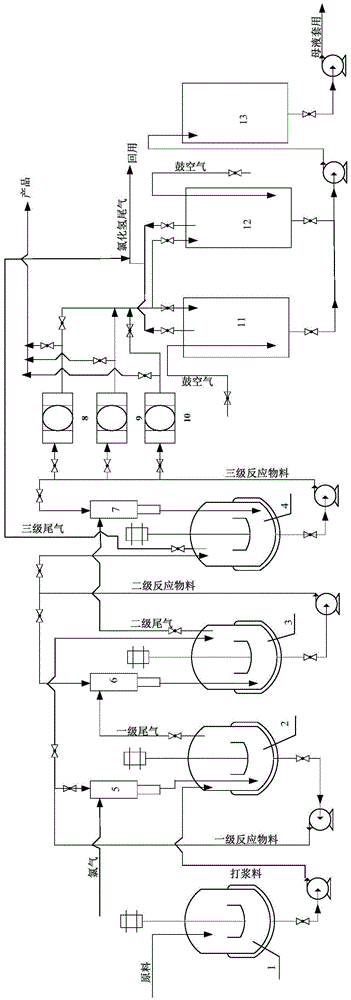
[0044] Such as figure 1 , using three-stage series reactors (2, 3, 4), three parallel filter presses (8, 9, 10) and two parallel mother liquor recovery tanks (11, 12), the first, second, and third stages The volume of the reactor is 50m 3 , control the chlorination temperature at 40-45°C, and the volume of the mother liquor recovery tank is 50m 3 , the volume of the filter press is 400m 3 , the volume of the mother liquor storage tank is 100m 3 , The liquid level of the mother liquid recovery tank is set to 90% of the volume.
[0045]In the beating kettle (1), feed raw materials simultaneously and continuously for beating, control the feed rate of p-nitroaniline to be 690kg / h, and the feed rate of 31% hydrochloric acid to be 7065kg / h, and the material in the beating kettle (1) Enter the primary reaction still (2) with the speed of 7755kg / h again; A part of the pump is self-circulating, and a part enters the secondary reactor (3) at a flow r...
Embodiment 2
[0050] According to the operation method described in Example 1, only the hydrochloric acid is replaced with the mother liquor in the mother liquor storage tank, and the filter cake from the filter press is washed and dried to obtain the 2,6-dichloro-p-nitro Aniline, measured purity (HPLC) 98.4%, yield 96.9%. The collected HCl gas is used for the synthesis of m-acetaniline, and the purity (HPLC) of 99% m-acetaniline can be obtained.
Embodiment 3
[0052] Continuous synthesis:
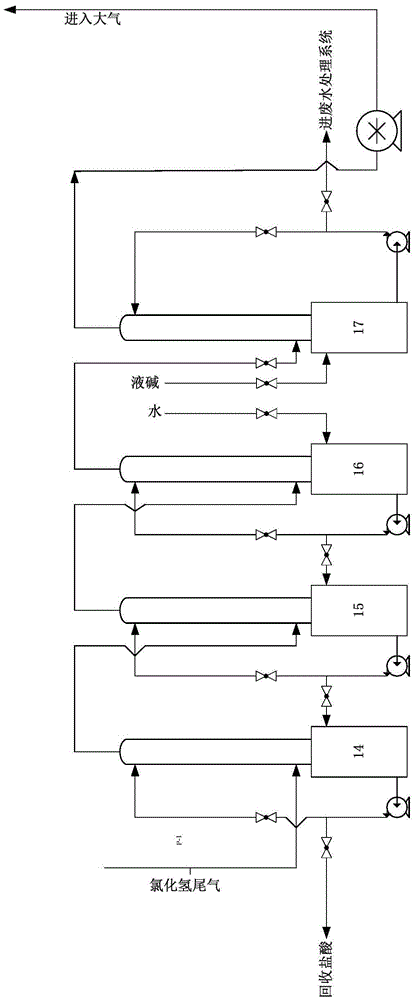
[0053] By the continuous synthesis operation method described in embodiment 1, the difference is the flow rate of raw materials: the feed rate of p-nitroaniline is 552kg / h, and the feed rate of 29% hydrochloric acid is 5790kg / h, the beating still The material enters the first-stage reactor at a rate of 6342kg / h; the chlorine gas is passed into the first-stage reactor at a flow rate of 525kg / h through the injector, and the material of the upper-stage reactor is passed into the next-stage reactor at a flow rate of 6867kg / h Reactor; then pumped into the filter press to filter at a flow rate of 6867kg / h, and the filter press also entered the mother liquor recovery tank at a flow rate of 6867kg / h; air was blown in the mother liquor recovery tank, and when the concentration of hydrochloric acid was 29%, the drum was stopped Air, the mother liquor enters the mother liquor storage tank, and the hydrogen chloride tail gas is collected at the same time; th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com