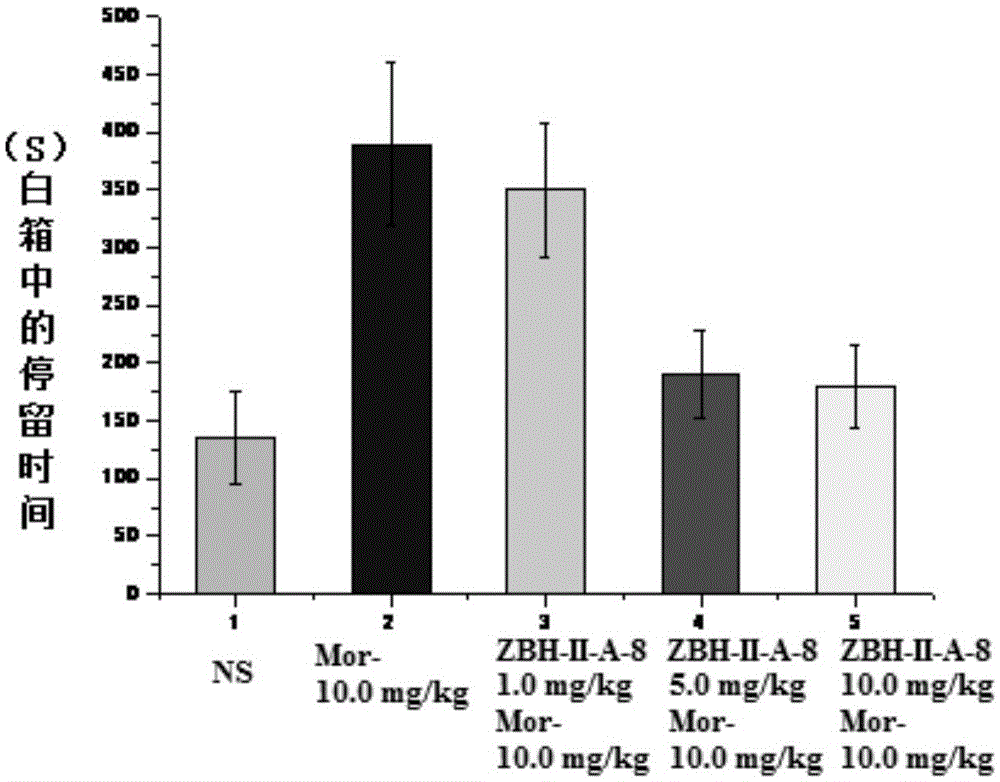
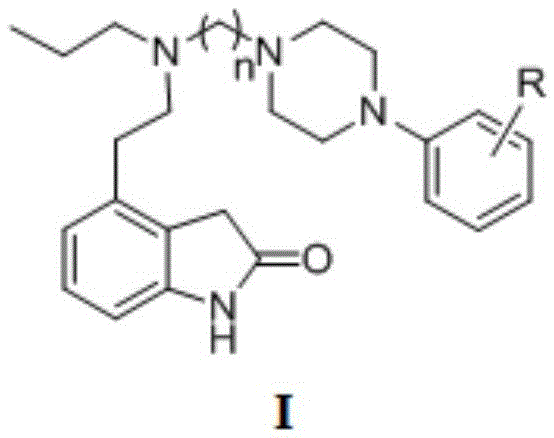
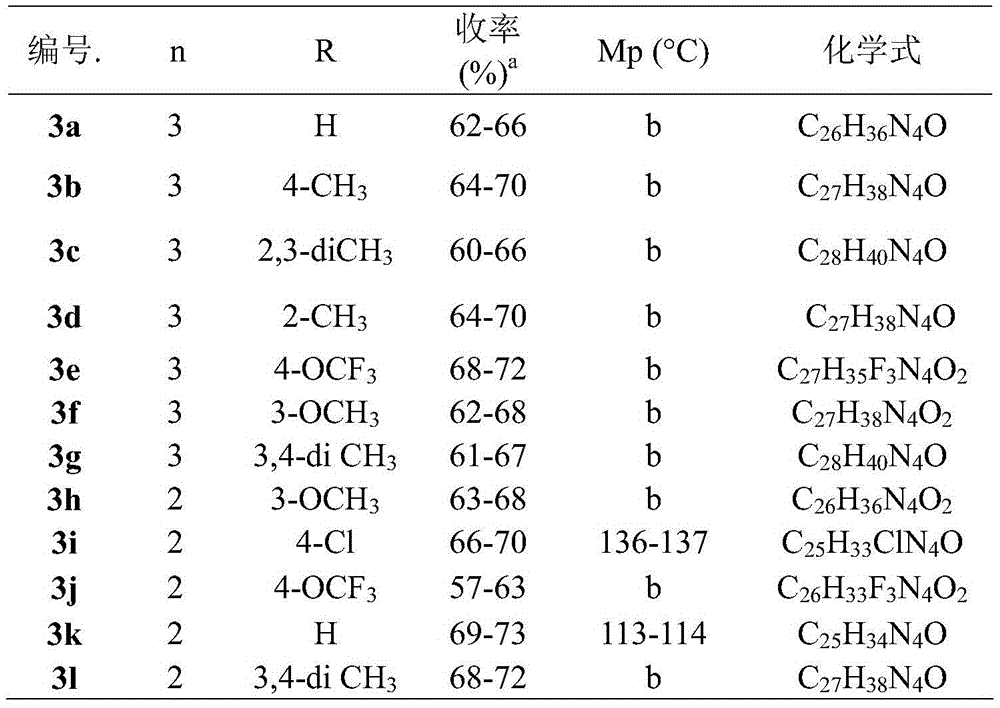
Indoline-2-ketone D3 receptor ligand and preparation method and application thereof
A kind of indoline and receptor technology, applied in the field of indoline-2-one D3 receptor ligand and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 14-(2-((3-(4-phenylpiperazin-1-yl) propyl) (propyl) amino) ethyl) preparation of indolin-2-one (3a) one, Preparation of 1-(3-chloropropyl)-4-phenylpiperazine (1a)
[0045] (1) Preparation of two (2-chloroethyl) amine hydrochloride (5)
[0046] Thionyl chloride (128 mL, 1.76 mol) was added into 80 mL of chloroform, stirred, and 68 mL of chloroform-diluted diethanolamine (40 mL, 0.417 mol) was slowly added dropwise within 1 h. After dropping, react at room temperature for 2-5 hours, slowly raise the temperature to 70° C., and end the reaction after reflux for 0.5-1 hour. Cool, filter with suction, wash the filter cake twice with dichloromethane, and dry to obtain 66 g of a white solid with a yield of 89% and a melting point of 214-215°C (207-209°C in literature).
[0047] (2) Preparation of 1-phenylpiperazine hydrochloride (7)
[0048] Compound 5 (30 g, 0.168 mol) was added to 150 mL of n-butanol, stirred, and aniline (14.25 g, 0.153 mol) diluted with 10 mL of n-...
Embodiment 2
[0076] Example 23-(3-(4-(4-chlorophenyl)piperazin-1-yl)propyl)-10-methoxymethyl-2,3,4,4a,5,6-hexahydro -1H-
[0077] Preparation of pyrazino[1,2-a]quinoline (3b)
[0078] Referring to the preparation method of compound 3a, compound 1b was prepared, and intermediate 2 (0.70 g, 3.21 mmol) was put in. After the reaction, 0.93 g of brown viscous compound was obtained, with a yield of 67%.
[0079] 1 HNMR (300MHz, CDCl 3 ),δ H ,ppm:0.88(t,J=7.2Hz,3H),1.41-1.53(m,2H),1.63-1.73(m,2H),2.26(s,3H),2.39(t,J=7.4Hz,2H ),2.46(t,J=7.5Hz,2H),2.54(t,J=7.3Hz,2H),2.60(s,4H),2.67(s,4H),3.15(s,4H),3.47(s ,2H),6.71(d,J=7.7Hz,1H),6.84(d,J=7.9Hz,3H),7.06(d,J=8.0Hz,2H),7.13(t,J=7.7Hz,1H ),9.28(s,1H); 13 C-NMR (300MHz, CDCl 3 ), δ: 11.9, 20.3, 20.3, 24.5, 30.9, 35.1, 49.6, 52.1, 53.2, 54.3, 56.1, 56.6, 107.5, 116.3, 122.7, 124.0, 127.9, 129.1, 129.5, 137.1, 142.5, 147.6, 17; IR(KBr,cm -1 ):3368,2954,2815,1687,1610,1577,1511,1459,1451,1237,811,720,651; MS:435.3[M+H] + .
Embodiment 3
[0080] Example 34-(2-((3-(4-(2,3-dimethylphenyl)piperazin-1-yl)propyl)(propyl)amino)ethyl)indoline-2 - Preparation of ketones (3c)
[0081] Referring to the preparation method of compound 3a, compound 1c was prepared, and intermediate 2 (0.70 g, 3.21 mmol) was added. After the reaction, 0.89 g of brown viscous compound was obtained, with a yield of 62%.
[0082] 1 HNMR (300MHz, CDCl 3 ),δ H ,ppm:0.89(t,J=7.3Hz,3H),1.43-1.55(m,2H),1.65-1.75(m,2H),2.22(s,3H),2.27(s,3H),2.39-2.49 (m,4H),2.53-2.69(m,10H),2.91-2.94(m,4H),3.48(s,2H),6.72(d,J=7.7Hz,1H),6.83-6.93(m,3H ), 7.07(t, J=7.7Hz, 1H), 7.15(t, J=7.7Hz, 1H), 9.08(s, 1H); 13 C-NMR (300MHz, CDCl 3 ), δ: 11.9, 13.9, 20.2, 20.5, 24.5, 30.8, 35.1, 52.0, 52.1, 53.7, 54.2, 56.0, 56.7, 107.5, 116.6, 122.7, 124.0, 124.8, 125.7, 127.9, 131.1, 137.1, 137 142.5, 151.5, 177.6; IR (KBr, cm -1 ):3440,3067,3025,2952,2872,1679,1619,1606,1581,1475,1460,1452,1376,1243,1145,1082,779,721,651; MS:449.4[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com