Olefin polymerization catalyst as well as preparation method and application method thereof
A catalyst and ethylene polymerization technology, applied in the direction of nickel organic compounds, etc., can solve the problems that late transition metal catalysts cannot meet the requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
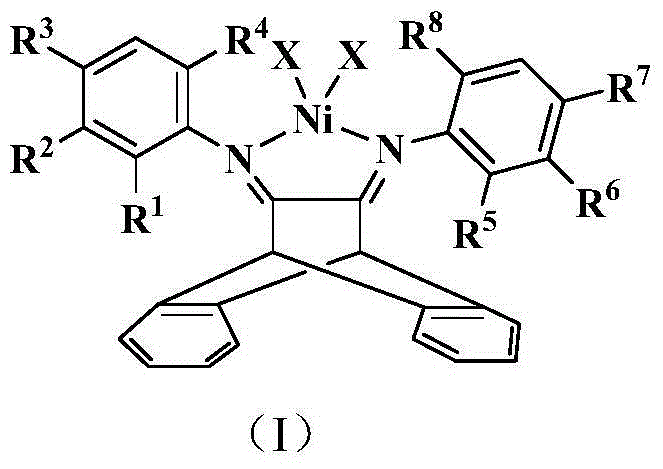
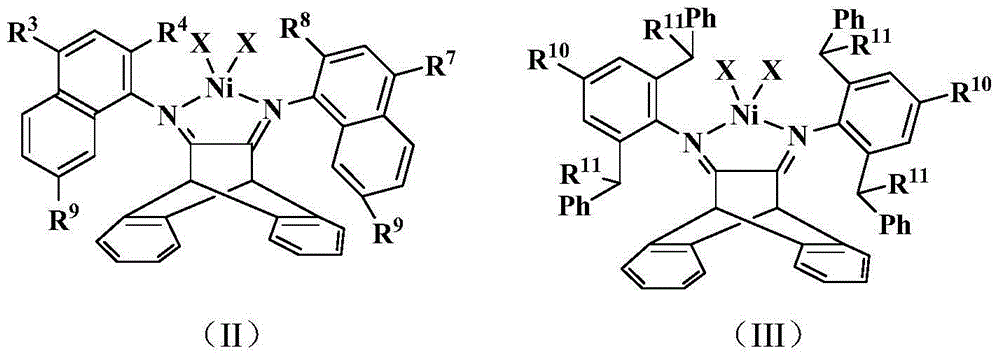
[0061] The compound of this embodiment is shown in structural formula (II), wherein R 4 , R 8 is methyl, R 3 , R 7 , R 9 is hydrogen, X is Br.
[0062] 1) Preparation of ligand:
[0063] 11,12-(9,10-dihydro-9,10-ethyleneanthracene)bis(2-methylnaphthylamine):
[0064] 9,10-dihydro-9,10-ethylene anthracene-11,12-dione (1.14g, 4.8mmol) and 2-methylnaphthylamine (1.63g, 10.4mmol), 0.5g p-toluenesulfonic acid is The catalyst was refluxed in 100mL toluene for 1 day, and the solvent was removed after filtration, and the residue was dissolved in dichloromethane. The perbasic alumina column was washed with petroleum ether / ethyl acetate (20:1), and the product was separated and removed. The solvent gave a yellow solid in 46% yield. 1 HNMR (CDCl 3 , δ, ppm): 2.46 (s, 6H), 4.86 (s, 2H), 7.05-7.36 (m, 14H), 7.62-7.72 (m, 6H).
[0065] 2) Preparation of complex 1: [11,12-(9,10-dihydro-9,10-ethyleneanthracene) bis(2-methylnaphthylamine)]nickel(II) bromide: mix 10ml (DME)NiBr 2 (27...
Embodiment 2
[0068] 10atm ethylene polymerization: Dry the 1L stainless steel polymerization kettle equipped with mechanical stirring at 130°C for 6hrs continuously, vacuumize while it is hot and use N 2 Air replacement 3 times. 6.6 mg (10 μmol) of complex 1 were added and then evacuated and replaced with ethylene 3 times. 500 ml of hexane was injected, and 6.5 ml of methylaluminoxane (MMAO) (1.53 mol / l toluene solution) was added to make Al / Ni=1000. At 90°C, the ethylene pressure was maintained at 10 atm, and the reaction was vigorously stirred for 30 min. Neutralize with ethanol solution acidified with 5% hydrochloric acid to obtain polyethylene with a polymerization activity of 6.28×10 6 g·mol -1 (Ni)·h -1 .
Embodiment 3
[0070] The compound of this embodiment is shown in structural formula (II), wherein R 3 , R 4 , R 7 , R 8 is methyl, R 9 is hydrogen, X is Br.
[0071] 1) Preparation of ligand: 11,12-(9,10-dihydro-9,10-ethylene anthracene) bis(2,4-dimethylnaphthylamine): 9,10-dihydro-9, 10-ethylene anthracene-11,12-dione (1.82g, 7.8mmol) and 2,4-dimethylnaphthylamine (2.98g, 17.4mmol), 0.5g p-toluenesulfonic acid as catalyst, in 100mL toluene Reflux for 1 day, remove the solvent after filtration, dissolve the residue with dichloromethane, wash with petroleum ether / ethyl acetate (20:1) over a basic alumina column, separate the product, remove the solvent to obtain a yellow solid, and produce The rate is 42%. 1 HNMR (CDCl 3 , δ, ppm): 2.45 (s, 6H), 2.67 (s, 6H), 4.86 (s, 2H), 7.05-7.34 (m, 14H), 7.60-7.71 (m, 4H).
[0072] 2) Preparation of complex 2: [11,12-(9,10-dihydro-9,10-ethyleneanthracene) bis(2,4-dimethylnaphthylamine)]nickel(II) bromide : 10ml (DME)NiBr 2(155mg, 0.5mmol) dich...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com