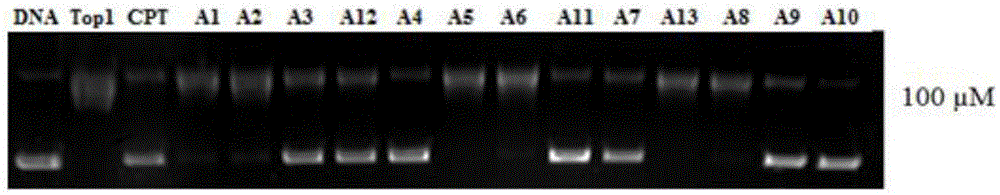
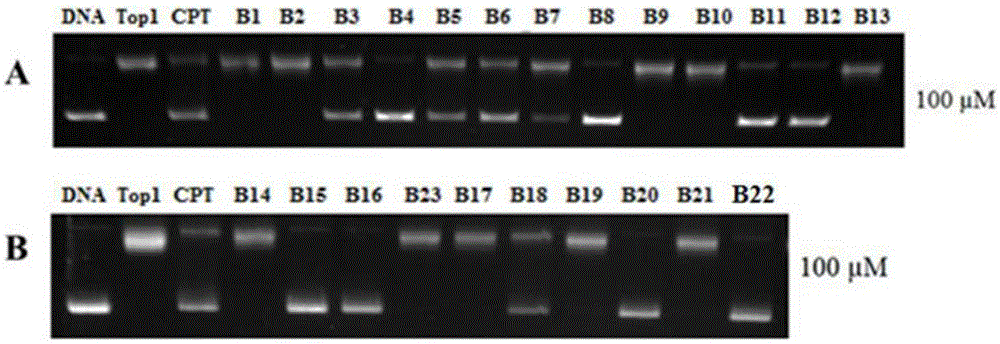
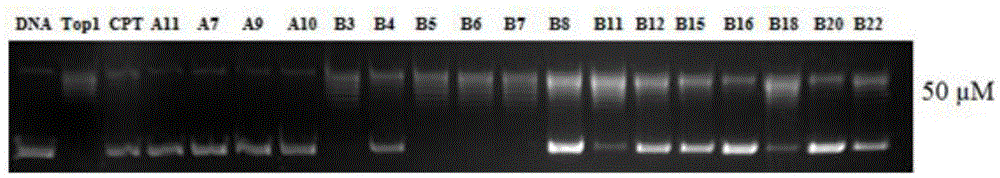
Multi-targeted antitumor active evodiamine derivative and preparation and application thereof
A technology of evodiamine and methoxy evodiamine, which is applied in the field of medicine and can solve the problems of complex pharmacokinetic properties of drug-drug interactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Preparation of 13-(7-(hydroxyamino)-7-oxoheptyl) evodiamine (A1)
[0110] NaH (60% oil, 1.2 mmol) was added into a DMF solution (10 mL) containing evodiamine (0.2 g, 0.66 mmol), and stirred at room temperature for 15 min. Bromo-7-heptanoic acid methyl ester (0.176g, 0.79mmol) was added, and the temperature was raised to 80°C to continue the reaction for 24h. TLC spot plate monitoring. After the reaction was completed, it was diluted with 50 mL of water, and extracted three times with 50 mL of ethyl acetate. The organic layers were combined, dried over anhydrous sodium sulfate, concentrated and column chromatographed (CH 2 Cl 2 : MeOH=100:1) to obtain the pure compound intermediate 13-(7-(methoxy)-7-oxoheptyl)evodiamine (5) (0.21g, yield 77%). Dissolve hydroxylamine hydrochloride (2.33g, 34mmol) in 12mL of methanol and stir in an ice bath, add methanol (7mL) dissolved in potassium hydroxide (2.81g, 50mmol), keep stirring at 0°C for 15min, add Magnesium su...
Embodiment 2
[0113] Example 2: Preparation of 10-hydroxyl-13-(4-(hydroxycarbamoyl)benzyl)evodiamine (A12)
[0114] Referring to Example 1, the product 13-(4-(hydroxycarbamoyl)benzyl)-10-methoxyevodiamine A3 was prepared.
[0115] Dissolve A3 (0.2g, 0.414mmol) in 10mL of dichloromethane, add BBr at -78°C under nitrogen protection 3 (0.5 mL), stirred for 30 min, then gradually raised to room temperature to continue the reaction for 2 h, after the reaction was completed, the reaction solution was washed with 50 mL of saturated aqueous sodium bicarbonate solution. Dichloromethane 50mL was extracted three times, the organic layers were combined, and dried with anhydrous sodium sulfate, the solvent was evaporated, and the residue was purified by column chromatography (CH 2 Cl 2 : MeOH=100:10) to obtain the target product A12 (0.071g, yield 35%).
[0116] 1 H-NMR (300MHz, DMSO-d 6 ,TMS):δ=11.12(s,1H),8.98(s.1H),8.89(s,1H),7.87(d,J=7.5Hz,1H),6.19(d,J=7.5Hz,2H) ,7.50(t,J=7.5Hz,1H),7.10-7.28(m...
Embodiment 3
[0118] Example 3: Class B compound intermediate 7(6-methoxy-3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)(phenyl)methanol Preparation of ketones
[0119] Compound 3 (4g, 19.98mmol) was dissolved in 100mL methanol, stirred at 0°C, and NaBH was slowly added 4 (0.83g, 21.97mmol) was reacted for 2h, monitored by TLC, after the reaction solution was distilled under reduced pressure, dissolved in 200mL of water, adjusted to pH 7-8 with dilute hydrochloric acid, a yellow solid was precipitated, filtered with suction, washed with water, and dried to obtain the intermediate Compound 4 (3.8 g, yield 91.0%). Compound 2 (2.5g, 12.38mmol), benzoic acid (1.66g, 13.61mmol), HBTU (5.6g, 14.86mmol), triethylamine (3.75g, 37mmol), dissolved in DMF (100ml), stirred at room temperature 2 hours, TLC monitoring. After the reaction was completed, the reaction solution was poured into 500ml of cold water, extracted three times with 200ml of ethyl acetate, the organic layers were combined, and washed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibitory activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com