Novel gall bladder contraction poison receptor antagonist analogue and synthetic method thereof
The technology of a receptor antagonist and a synthesis method is applied in the synthesis of novel aspenisin analogs and the field of synthesis thereof, which can solve the problems of inconvenience in taking, short half-life and the like, and achieve a short route, simple separation and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
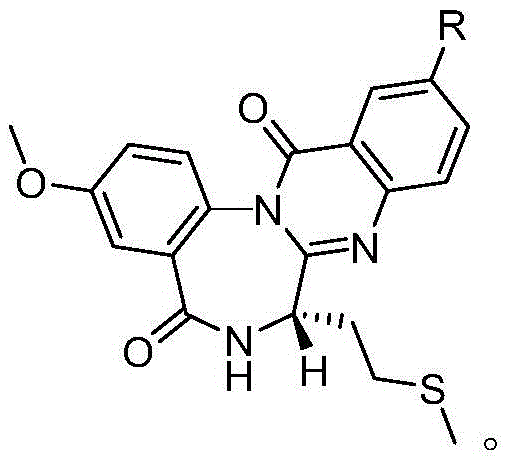
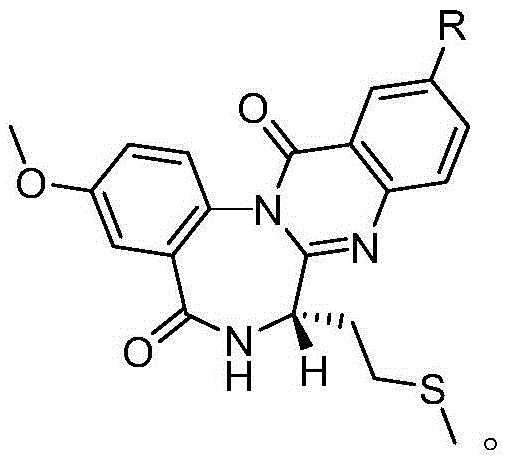
[0026] A novel cholecystokinin receptor antagonist analog, which has the following structure:
[0027]
[0028] Example 1
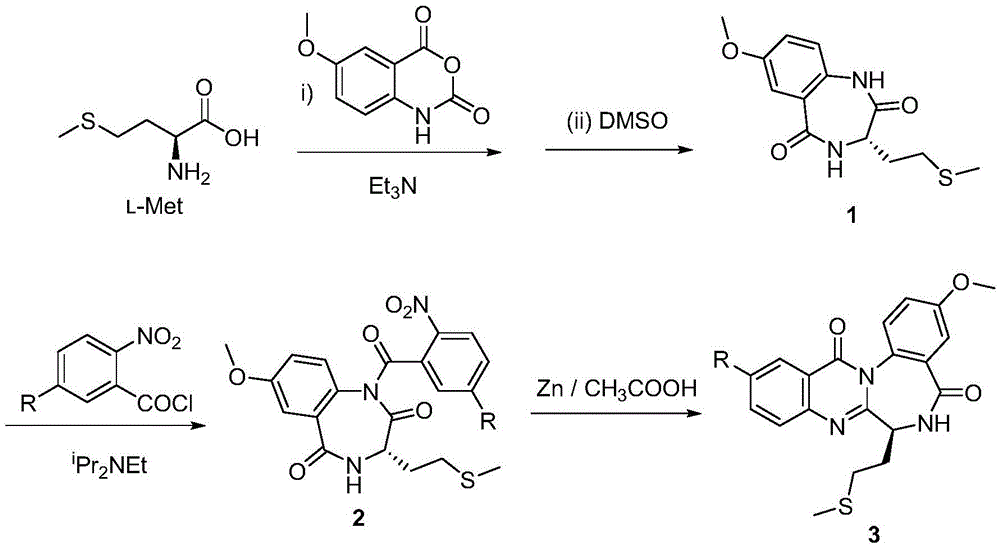
[0029] A synthetic method of a novel cholecystokinin receptor antagonist analog, comprising the following steps:
[0030] 1) At room temperature, dissolve 5-methoxyisatoic acid rod (5.79g, 30mmol) in an aqueous solution of triethylamine (triethylamine 4.2mL, water 30mL) to make a 1.0mol / L solution, and then add L-Methionine (4.5g, 30mmol), the reactant was stirred at room temperature for 5 hours, concentrated under reduced pressure to remove water, and pumped to dryness. The intermediate was directly used as the raw material for the next step without separation and purification.
[0031] 2) Add the intermediate in the previous step into DMSO (1000mL) and heat and stir at 135-150°C for 5 hours, then cool to 0°C, add 1000mL of ice water under cooling, and extract with ether (150mL×6), combine organic phase, washed with saturated sodium chloride solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com