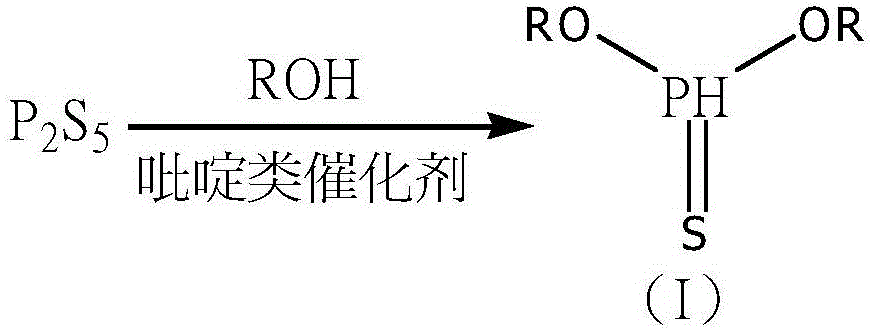
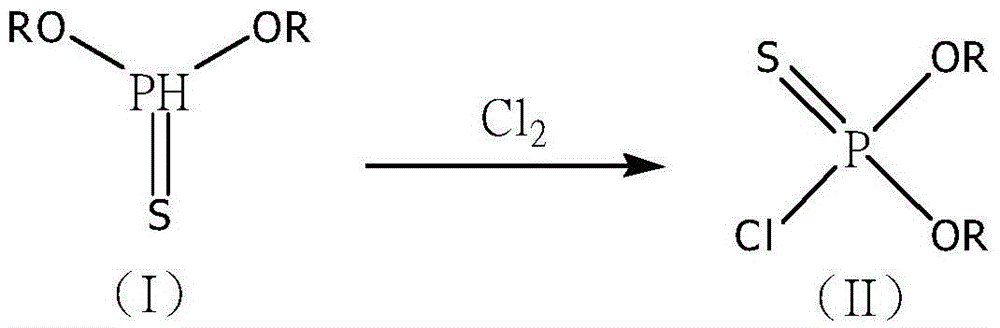
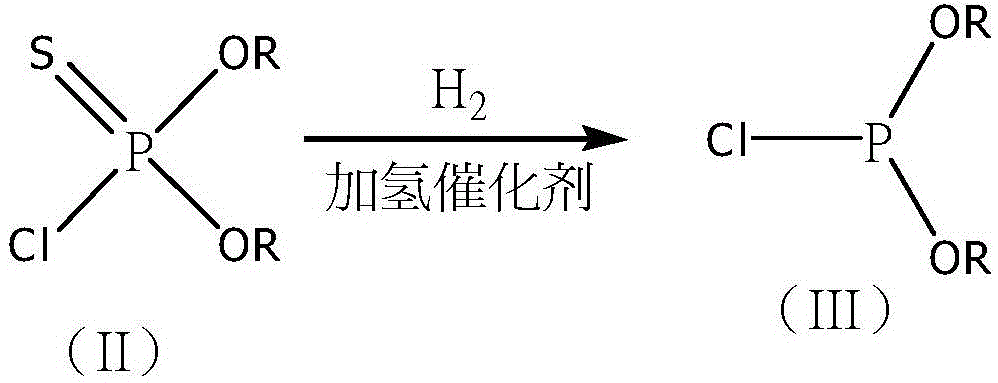
Synthetic method of methyl phosphite and glufosinate-ammonium
A technology of methyl phosphonite and synthesis method, which is applied in the synthesis field of organophosphorus compounds, can solve the problems of high requirements, potential safety hazards, personal injury of operators, etc., and achieves improvement of synthesis yield, reduction of emissions, and reduction of environmental protection. Effects of cost and pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 111.1gP in a 500ml four-neck flask 2 S 5 (0.25mol) and 1.2gDMAP (0.01mol), add 46g ethanol (1mol) in the constant pressure titration funnel, when the temperature rises to 70 ℃, start to drop ethanol, and control the reaction temperature at 60-100 ℃, when the specific gravity of the material liquid reaches At 1.170-1.180, the dropwise addition is completed, keep warm for 20-30min, and start to cool down to obtain the crude sulfide (I).
[0030] When the temperature of the sulfide crude product (I) dropped to 35°C, pass 71g of chlorine gas (1mol) to carry out the chlorination reaction, control the flow of chlorine gas, absorb the tail gas with 1000g of deionized water, measure the specific gravity of the material and liquid every 2 hours, and control the chlorination The temperature is between 35-50°C, and the reaction is stopped when the specific gravity of the reaction solution is 1.275-1.285, and the crude chloride (II) is obtained.
[0031] Add dropwise 19.5gNa ...
Embodiment 2
[0035] The method is the same as in Example 1, and the specific parameters are shown in Table 1, Table 2, and Table 3. The distilled refined chlorophosphonite is directly used as a hydrogenation raw material in a fixed-bed reactor for catalytic reaction. Use a tubular reactor with an inner diameter of 32mm, load 20g of metal catalyst (20-40 mesh), the active components are Ni, Cu, Pt, the additives are Zr, Ce, and the carrier is γ-Al 2 o 3 .
Embodiment 3
[0037] The method is the same as in Example 1, and the specific parameters are shown in Table 1, Table 2, and Table 3. The distilled refined chlorophosphonite is directly used as a hydrogenation raw material in a fixed-bed reactor for catalytic reaction. A tubular reactor with an inner diameter of 32 mm was used to load 20 g of metal catalyst (20-40 mesh), the active components of which were Rh and Pt, the auxiliary agents were Y and La, and the carrier was 5A molecular sieve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com