Nasal mucosal vaccine composition
一种疫苗组合物、鼻粘膜的技术,应用在药物组合、微生物、药物输送等方向,能够解决不明确、难以防御感染自身等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5
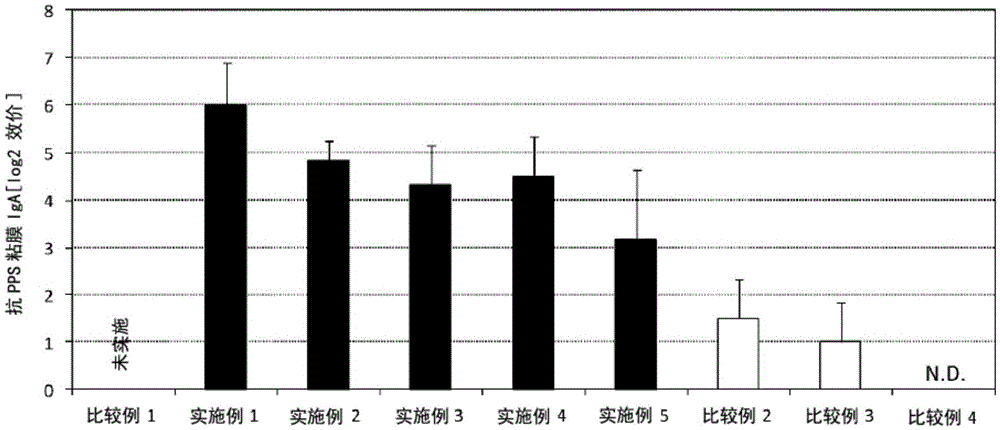
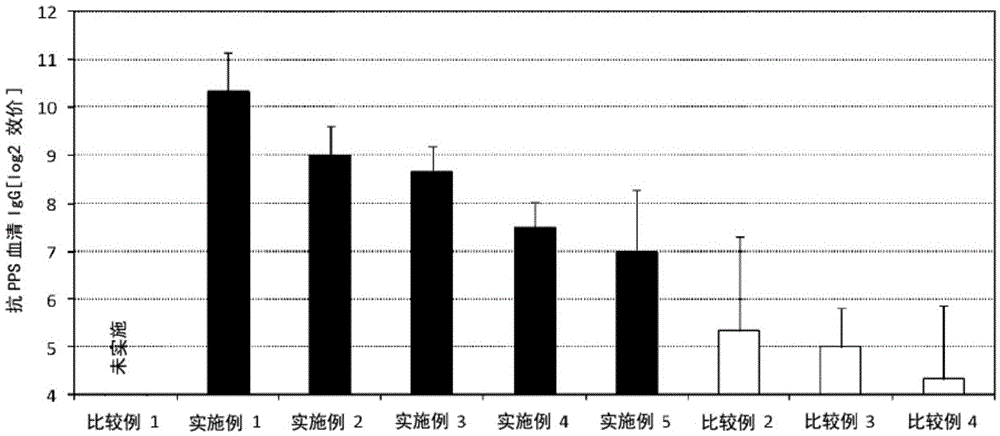
[0072] (Examples 1-5, Comparative Examples 1-4)
[0073] Each administration group was manufactured with 10 parts.
[0074] A solution (1150 μg / mL) containing pneumococcal capsular polysaccharide (Pneumovax NP, manufactured by MSD Co., Ltd.) and lipopolysaccharide derived from Pantoea agglomerans (Natural Immunity Using the solution (50 mg / mL, manufactured by Giken Co., Ltd.), phosphate buffer solution (manufactured by NACALAITESQUE, INC.) was added to prepare 100 µL of a vaccine composition. For example, in Example 1, 8.7 μL of a solution containing pneumococcal capsular polysaccharide was added, 20 μL of a solution of lipopolysaccharide derived from Pantoea agglomerans (Pantoea agglomerans) was added, and then phosphate buffer was added to make the total amount 100 μL. Other Examples and Comparative Examples were also appropriately diluted to form a content equivalent to the dosage. In Comparative Example 4, only phosphate buffered saline (NACALAITESQUE, INC. .system).
...
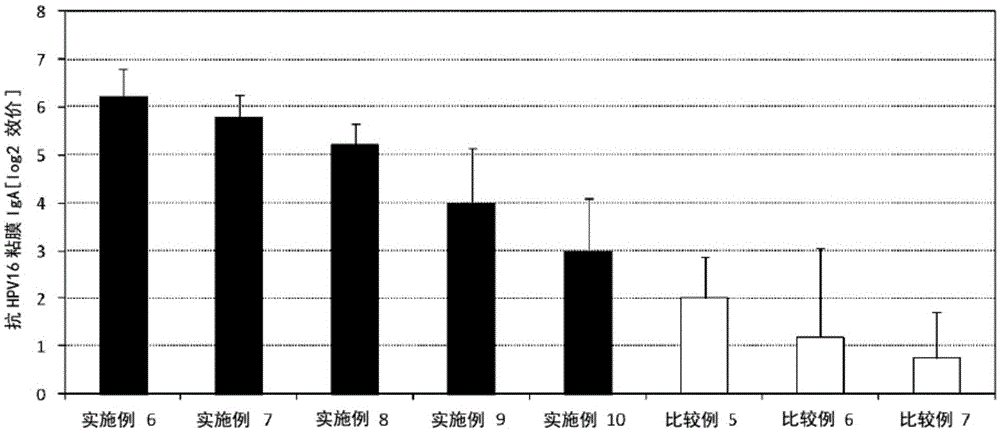
Embodiment 6~10、 comparative example 5~7
[0081] Except that the solution containing pneumococcal capsular polysaccharide was changed to a solution containing HPV16 recombinant protein (HPV16, produced by PROSPEC Co., Ltd.) (820 μg / mL), basically based on Examples 1 to 5 and Comparative Examples 1 to 4, The procedure produced vaccine compositions corresponding to Table 2. For example, in Example 6, after adding 12.2 μL of the solution containing the HPV16 recombinant protein and 20 μL of the lipopolysaccharide solution derived from Pantoea agglomerans (Pantoea agglomerans), phosphate buffer was added so that the total amount was 100 μL.
[0082] Six mice (8-week-old female BALB / C mice, Japan SLC, Inc.) were anesthetized, and 10 μL of the prepared vaccine composition was nasally administered to each mouse. One week after the administration, the mice were anesthetized again, and 10 μL of the prepared vaccine composition was nasally administered to each mouse. After the second administration and one week later, the seru...
Embodiment 11~13、 comparative example 8
[0086] 50 μL of a solution containing attenuated surviving rotavirus (RotaTeq internal solution, manufactured by MSD Corporation) was added, and 50 μL of a solution of lipopolysaccharide (manufactured by NACALAITESQUE, INC.) derived from Pantoea agglomerans (Pantoea agglomerans) was added in Example 11 ( 2 mg / mL), in Example 12, 5 μL of lipopolysaccharide (NACALAITESQUE, INC.) solution derived from Pantoea agglomerans (Pantoea agglomerans) was added, and in Example 13, lipopolysaccharide derived from Pantoea agglomerans (Pantoea agglomerans) was added Polysaccharide (manufactured by NACALAITESQUE, INC.) solution 0.5 μL, 5 μL of glucopyranosyllipid (Glucopyranosyllipid) (MPLAs, manufactured by InvivoGen company) solution (2 mg / mL) was added in Comparative Example 8, phosphate buffer saline (NACALAITESQUE, INC. . system), thereby producing 100 μL of the vaccine composition. Six mice (8-week-old female BALB / C mice, Japan SLC, Inc.) were anesthetized, and 10 μL of the prepared vac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com