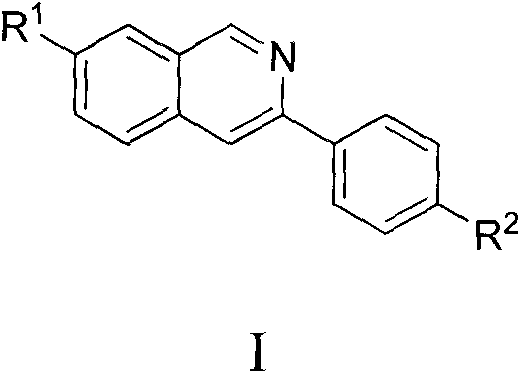
Isoquinoline compound and synthetic method thereof
A synthetic method and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of imperfect synthetic method, cumbersome post-treatment process, large catalyst loading, etc., and achieve the effect of simple synthetic method, high yield and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of compounds of the present invention
[0031] The compound preparation route of the present invention is as follows:
[0032]
[0033] 3a, 4a, 5a: R 1 = Me,R 2 =Me3b,4b,5b:R 1 = Me,R 2 = Cl3c, 4c, 5c: R 1 = Cl, R 2 = Me
[0034] 3d, 4d, 5d: R 1 = Cl, R 2 = Cl3e, 4e, 5e: R 1 = H, R 2 =n-Bu
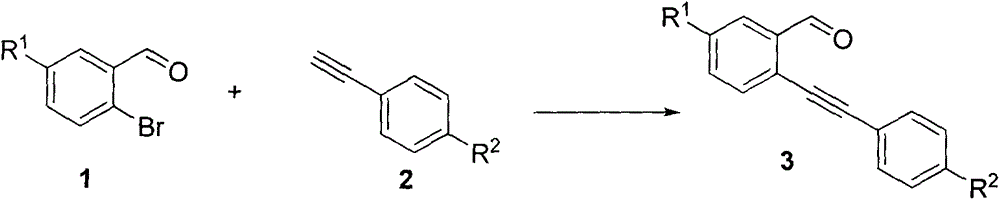
[0035] (1) Synthesis of intermediate compound 3a-3i:
[0036] Take 1mmol of compound 1a-1c, 1mmol of compound 2a-2c, 0.1mmol of CuI, 0.03mmol of Pd(PPh 3 ) 2 Cl 2 and 2mmol of triethylamine were dissolved in 5mL of tetrahydrofuran, under the protection of nitrogen, heated to reflux, and TLC monitored the reaction process. After the reaction was complete, the reactant was cooled to room temperature, an appropriate amount of water was added, and then separated and extracted, dried, and then carried out Column chromatography gave compounds 3a-3e.
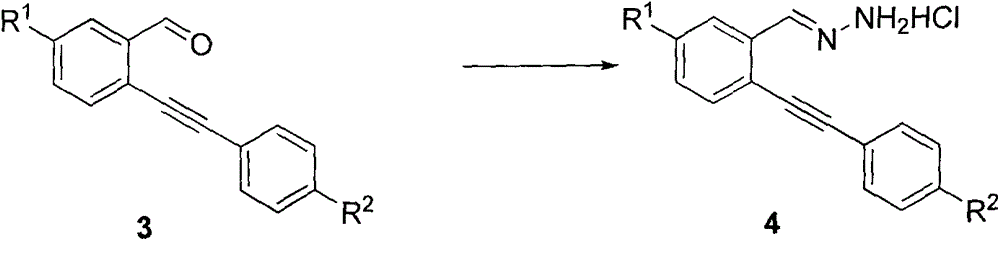
[0037] (2) Synthesis of intermediate compound 4a-4e:
[0038] Dissolve compound 3a-3e and hydrazine hydrate o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com