Hydrogen peroxide producing efficient oxidation method
A hydrogen peroxide, high-efficiency technology, applied in chemical instruments and methods, peroxide/peroxy hydrate/peroxyacid/superoxide/ozonide, inorganic chemistry, etc., can solve the long time of complete oxidation reaction and the safety of existence Hidden dangers, high tail oxygen content, etc., to reduce hydrogen peroxide decomposition and oxidation side reactions, increase gas-liquid reaction rate, and reduce oxidation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
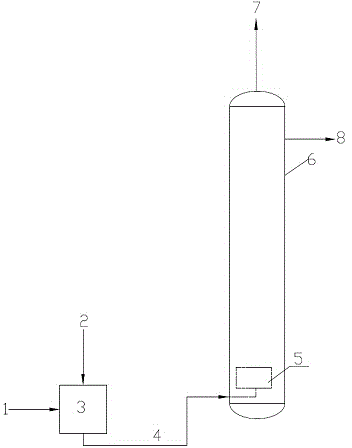
[0027] One section of oxidation tower, tower diameter 1200mm, height 2500mm, total volume of oxidation tower is 3.37m 3 . Hydrogenated liquid 60m 3 / h, the amount of air added is 2000Nm 3 / h. First mix the air and hydrogenated liquid with the pipeline, then introduce the high-pressure dissolved air pump under the conditions of 50-55 °C and 15-16 MPa, dissolve 40% of the air in the hydrogenated liquid, and then introduce the hydrogenated liquid into the atomizer, which is equipped with Atomizing nozzles can instantly atomize the gas-liquid mixture in the oxidation tower, and the atomized material undergoes oxidation reaction and then undergoes gas-liquid separation at the top of the tower. The results show that when the residence time of the reaction materials in the tower is 2.5-2.8 minutes, the oxidation yield after oxidation reaction is 95%-97%, and the tail oxygen content is 3%-5%.
Embodiment 2
[0029] Three identical oxidation towers are connected in parallel, the diameter of the tower is 600mm, the height is 3400mm, and the volume of a single oxidation tower is 1.01m 3 . Hydrogenation solution 3.1m 3 / h, the amount of air added 100Nm 3 / h. First, the air and the hydrogenation liquid are divided into three materials respectively, and after the gas-liquid mixing, three high-pressure dissolved air pumps are introduced under the conditions of 50-55°C and 8-10MPa to dissolve 32% of the air in the hydrogenation liquid, and then the gas-liquid The mixture is introduced into the atomizer, which is equipped with an atomizing nozzle, so that the gas-liquid mixture is atomized instantly in the oxidation tower, and the atomized material undergoes oxidation reaction and then undergoes gas-liquid separation at the top of the tower. The results show that when the residence time of the reaction materials in the tower is 2.2 to 2.4 minutes, the oxidation yield after oxidation rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com