Method for synthesizing betaine from long-chain tertiary amine by means of anhydrous quaternization under normal pressures
A long-chain tertiary amine and betaine technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of potential safety hazards and high equipment requirements, achieve high flash point, excellent performance, and inhibit hydrolysis The effect of product formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 10
[0026] Example 1 Synthesis of octadecyl dimethyl hydroxypropyl sultaine under normal pressure anhydrous quaternization
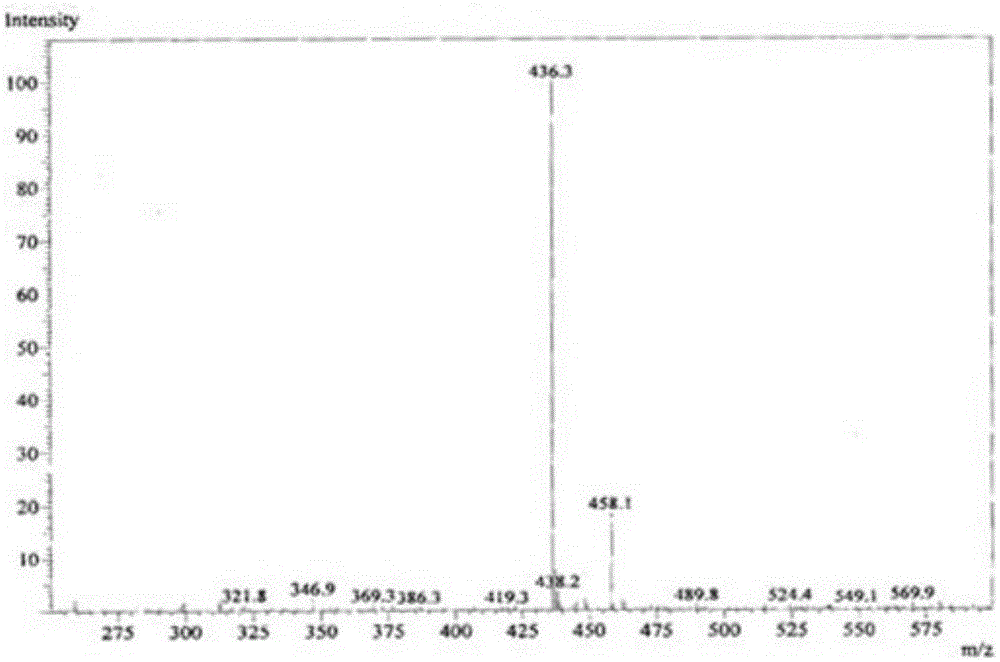
[0027] In a 250mL three-necked flask equipped with a thermometer and a condensing reflux tube, add 29.7g of 0.1mol octadecyldimethyl tertiary amine, 21.6g of 0.11mol sodium trichlorodihydroxypropanesulfonate, 10mL ethylene glycol, 10mL1,2, -Propylene glycol, 5mL n-butanol, then place the three-necked flask in an oil bath at 125°C, turn on the magnetic stirrer, and detect the reaction by measuring the residual amine value. After 3 hours, the measured amine value is converted to a conversion rate of greater than 96%, closed flash point It is 75°C. figure 1 It is the ESI-MS spectrum of the product. The molecular ion peak at 436 m / z is the +H peak of octadecyl dimethyl hydroxypropyl sultaine (Mw 435).
Embodiment 2
[0028] Example 2 Synthesis of erucic acid acyl propyl dimethyl hydroxypropyl sultaine under normal pressure anhydrous quaternization
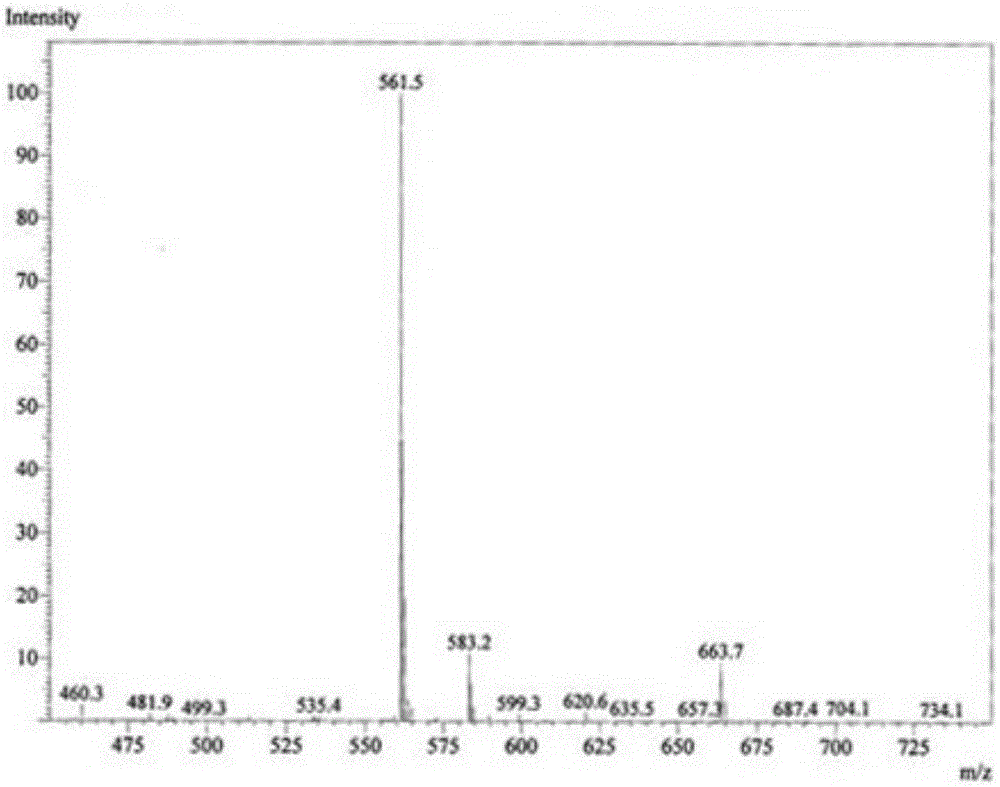
[0029] In a 250mL three-necked flask equipped with a thermometer and a condensing reflux tube, add 42.2g of 0.1mol octadecyldimethyl tertiary amine, 23.6g of 0.12mol sodium trichlorodihydroxypropanesulfonate, 10mL ethylene glycol, 15mL1,2. -Propylene glycol, 3mL n-pentanol, then place the three-necked flask in an oil bath at 135°C, turn on the magnetic stirrer, and detect the reaction by measuring the residual amine value. After 2.5 hours, the conversion rate of the measured amine value is greater than 95%, and the closed flash point It is 82°C. figure 2 It is the ESI-MS spectrum of the product. The molecular ion peak at 561 m / z is the +H peak of erucyl propyl dimethyl hydroxypropyl sulfobetaine (Mw 560).
Embodiment 3
[0030] Example 3 Synthesis of m-xylyl octadecyl dimethyl carboxy betaine under normal pressure anhydrous quaternization
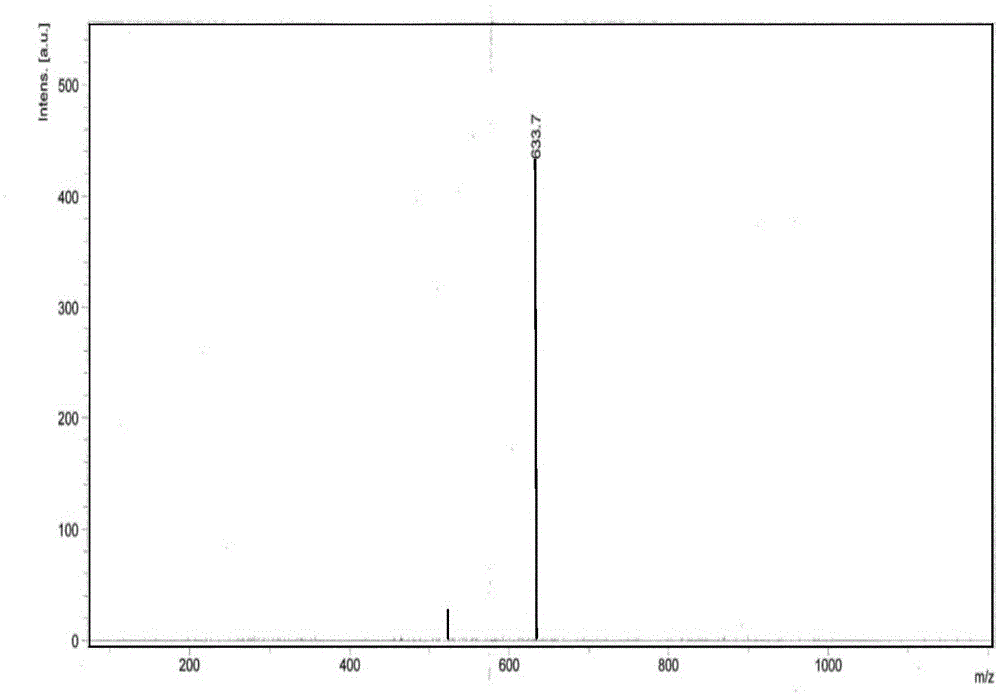
[0031] In a 250mL three-necked flask equipped with a thermometer and a reflux tube, add 0.1 mol of m-xylyl octadecyl dimethyl tertiary amine 40.1 g, 0.13 mol sodium chloroacetate 15.2 g, 10 mL ethylene glycol, and 20 mL 1,2-propylene glycol. , 4mL n-butanol, then place the three-necked flask in a 140℃ oil bath, turn on the magnetic stirrer, and detect the reaction by measuring the residual amine value. After 3.5 hours, the conversion rate of the measured amine value is greater than 98%, and the closed flash point is 68 ℃. image 3 It is the ESI-MS spectrum of the product. The molecular ion peak at 460 m / z is the +H peak of meta-xylyl octadecyl dimethyl carboxy betaine (Mw 459).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com