OFF-ON type TBT-detecting fluorescent probe and preparation method and application thereof
A compound and general formula technology, applied in the OFF-ON type detection TBT fluorescent probe and its preparation and application fields, can solve the problems of non-target biological ecological hazards, human health hazards, aquatic biological hazards, etc., and achieve high selective identification Ability and anti-interference ability, good biological application prospects, high selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of probes 2a-2c:
[0048]
[0049] a. Synthesis of rhodamine B hydrazide: Rhodamine B (25 g, 56.4 mmol) and 200 mL of absolute ethanol were added to a 500 mL three-necked flask, and excess 60 mL of hydrazine hydrate was added dropwise. The reaction solution was vigorously stirred and refluxed for 24 hours, and the dark red suspension gradually became clear. After the reaction was completed, the temperature was lowered and poured into 500 mL of water, a large amount of light yellow precipitate was produced immediately. Static suction filtration, the solid was washed with a small amount of water and absolute ethanol until it was colorless. The crude product was recrystallized from ethanol and dried.
[0050] Rhodamine B hydrazide: 20.40 g of white solid, yield: 80.2%.
[0051] 1 HNMR (CDCl 3 ,400MHz,TMS):δ(ppm)8.00–7.89(m,1H),7.53–7.40(m,2H),7.17–7.05(m,1H),6.55–6.37(m,4H),6.29(d, J=8.4Hz,2H),3.61(s,2H),3.34(dd,J=13.8Hz,6.8,8H),1.37–1.01(m,12...
Embodiment 2
[0067] Example 2: Synthesis of probes 3a-3c:
[0068]
[0069] a. Synthesis of rhodamine 6G hydrazide: add rhodamine 6G (25g, 60.2mmol) and 200mL absolute ethanol into a 500mL three-necked flask, and add dropwise excess 50mL of hydrazine hydrate. The reaction solution was vigorously stirred and refluxed for 24 hours, and the dark orange-yellow suspension gradually became clear. After the reaction was completed, the temperature was lowered and poured into 500 mL of water, a large amount of light yellow precipitate was produced immediately. Stand still and filter with suction, and wash the solid with a small amount of water and absolute ethanol until it is colorless. The crude product was recrystallized from ethanol and dried.
[0070] Rhodamine 6G hydrazide: 19.46 g of colored solid. Yield: 76.4%.
[0071] 1 HNMR (CDCl 3 ,400MHz,TMS):δ(ppm)7.96(dd,J=5.9Hz,2.7,1H),7.52–7.41(m,2H),7.15–7.02(m,1H),6.40(s,2H),6.26 (s,2H),3.58(s,2H),3.22(q,J=7.1Hz,4H),1.92(s,6H),1.33(t,J=7...
Embodiment 3
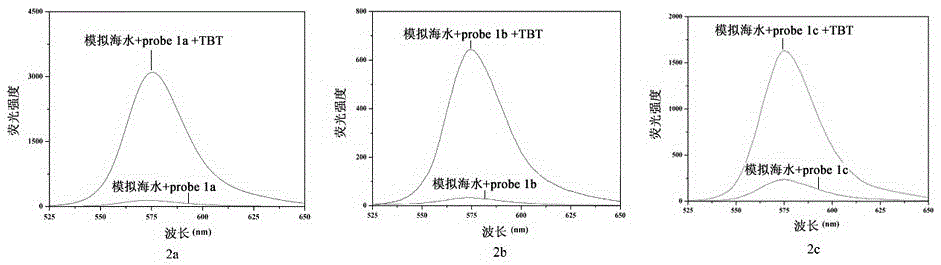
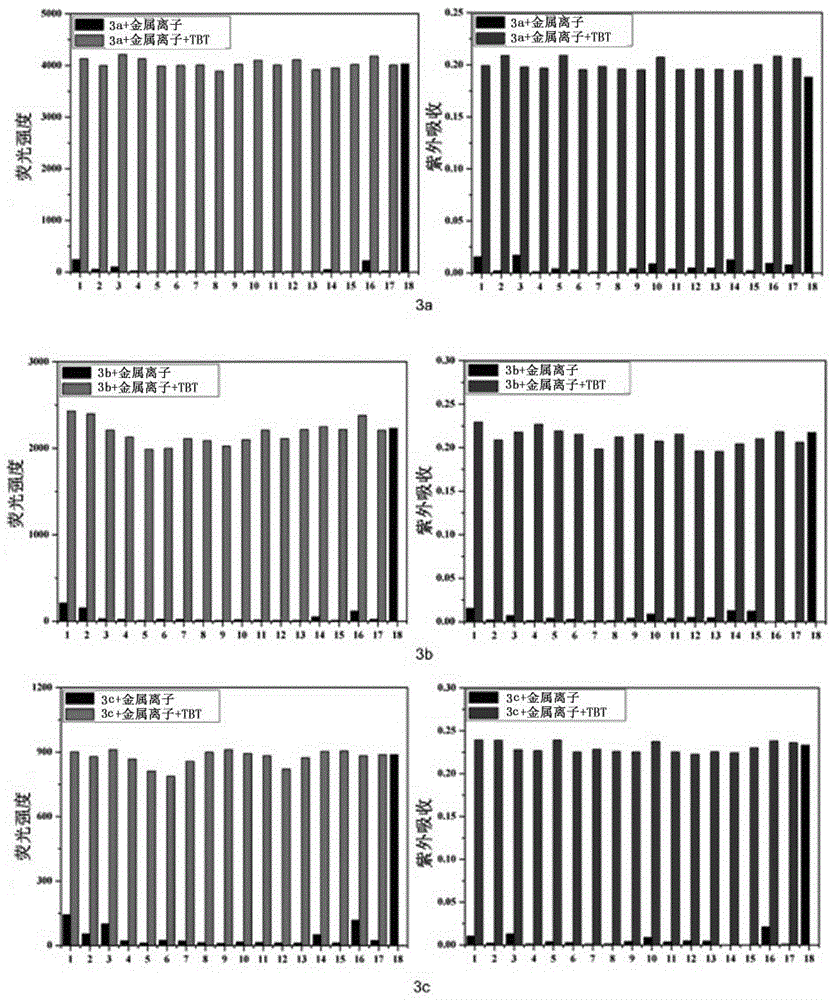
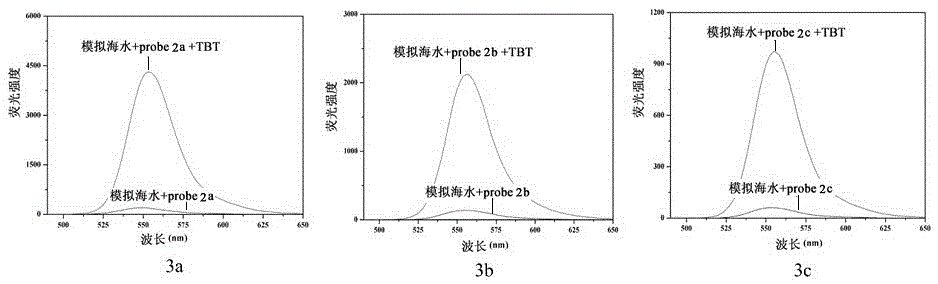
[0087] Embodiment 3: Probe 2a-2c and 3a-3c recognize the fluorescence and absorption data and spectrometry of TBT
[0088] Prepare a probe solution with a concentration of 5 μmol / L, and gradually add TBT solution for titration experiments. figure 1 is the optical data (excitation wavelength, maximum emission wavelength, fluorescence quantum yield, response time) of probes 2a-2c and 3a-3c. It can be seen that the synthesized probes have significant fluorescence emission in the range of 550-580nm, and The response time is extremely short, and the TBT content in the solution can be detected immediately. Figure 9 The fluorescence spectrum of probe 2a was titrated for TBT. It can be seen from the results that the probe itself has no fluorescence due to the existence of the lactone structure. For probe 2a, when the fixed concentration was 5μmol / L, with the addition of TBT, the fluorescence intensity at 577nm was gradually enhanced to 207 times of the original intensity. When the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com