Method for preparing ketone compound through biomimetic catalysis
A ketone compound, biomimetic catalysis technology, applied in the oxidation preparation of carbonyl compounds, oxidation reaction preparation, chemical instruments and methods, etc., can solve problems such as low conversion efficiency, achieve high selectivity, reduce energy consumption, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
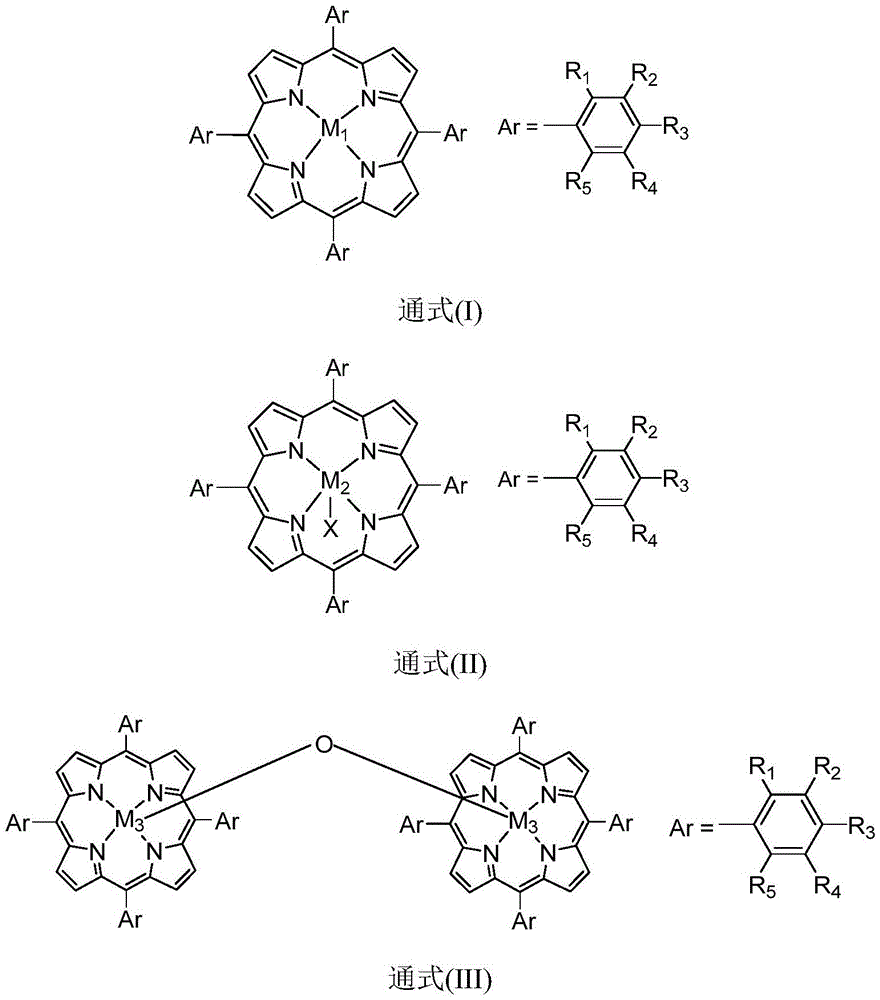
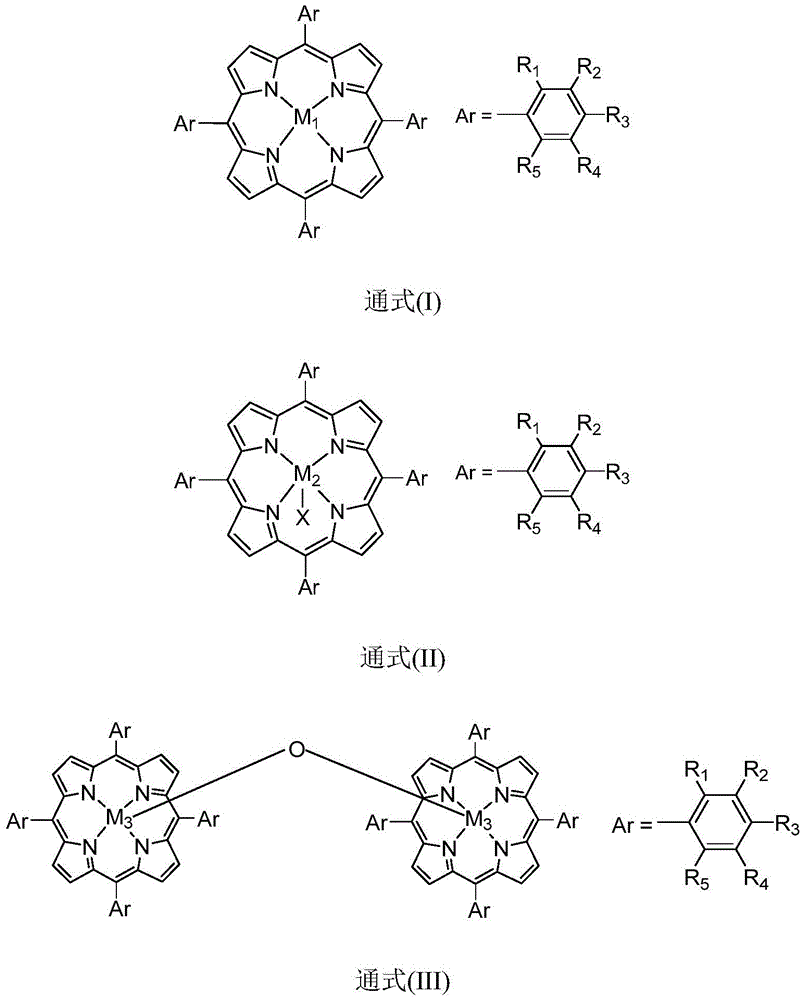
[0021] In the reactor, add the diphenylmethane of 10mmol and the isopropyl benzene of 20mmol, add 200ppm and have general formula (I) structure metalloporphyrin catalyst (M 1 =Fe,R 1 =Cl,R 2 =R 3 =R 4 =R 5 =H), the stirring reaction was carried out at a temperature of 40° C. under normal pressure. According to gas chromatography analysis, the conversion rate of raw materials was 90%, and the selectivity of product ketone was 98%.
Embodiment 2
[0023] In the reactor, add the ethylbenzene of 10mmol and the diisopropylbenzene of 100mmol, add 0.5ppm and have general formula (I) structure metalloporphyrin catalyst (M 1 =Co,R 2 = NO 2 , R 1 =R 3 =R 4 =R 5 =H), the stirring reaction was carried out at a temperature of 90° C. under normal pressure. According to gas chromatography analysis, the conversion rate of raw materials was 95%, and the selectivity of product ketone was 98%.
Embodiment 3
[0025] In the reactor, add the cyclohexane of 10mmol and the 1,3-diisopropylbenzene of 10mmol, add 10ppm and have general formula (I) structure metalloporphyrin catalyst (M 1 =Mn,R 3 =OCH 3 , R 1 =R 2 =R 4 =R 5 =H), the stirring reaction was carried out at a temperature of 50° C. under normal pressure. According to gas chromatography analysis, the conversion rate of the raw material was 92%, and the selectivity of the product ketone was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com