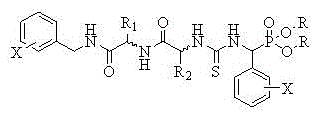
Chirality oliopeptide phosphonate thiourea derivatives and application and preparation method thereof
A technology of thiourea derivatives and peptide phosphonates, applied in the field of chemistry, can solve problems such as unreported synthesis methods, and achieve the effects of good symmetry selectivity, good reliability and simple sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
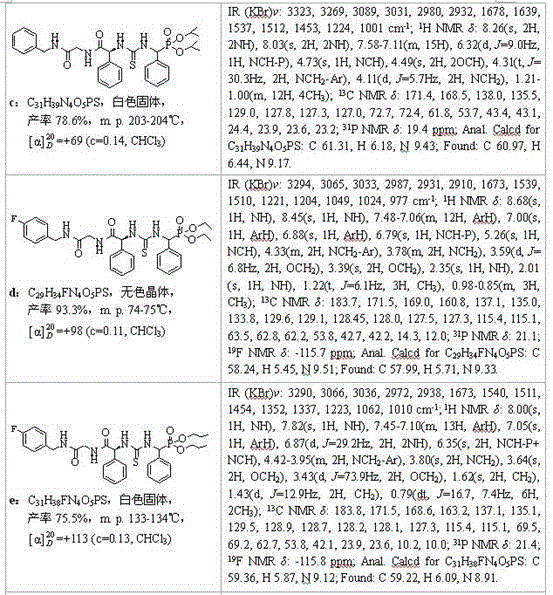
Embodiment 1
[0033] Example 1 of the present invention: Synthesis of compound O,O'-diethyl (3-(benzylamino-glycine-L-phenylglycine)-α-thioureido) phenylmethyl phosphate (compound number a):
[0034] (1) Synthesis of O,O'-diethylamino (phenyl) methyl phosphate:
[0035] Benzaldehyde (30mmol, 3.18g) and ammonia (28%, 60mL) were added dropwise to a 100mL dry three-necked flask, and stirred at room temperature for 3h. During this process, white precipitates are produced, which are filtered, washed with dilute ammonia and dried. Under reflux at 75°C and stirring for the reaction, the white solid was transferred to a dry three-necked flask containing diethyl phosphite (15mmol, 2.08g). The solid slowly dissolved, and an intermediate was formed after 8 hours of reaction. Then a mixture of p-toluenesulfonic acid (10mmol, 1.72g) and 50mL of THF was added dropwise to this intermediate, and the reaction was stirred for 10h at below 0°C. A large amount of white solid is generated, filter it, and wash the ...
Embodiment 2
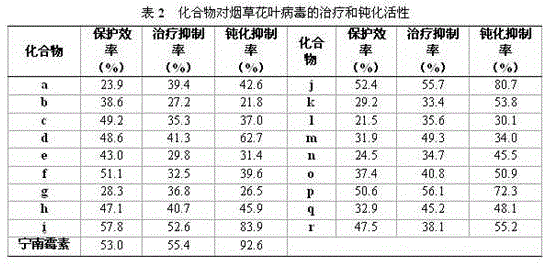
[0054] Example 2 of the present invention: The compound's inhibitory activity test against tobacco mosaic virus (TMV):
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com