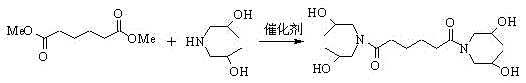Method for synthesis of N,N,N',N'-tetra(beta-hydroxypropyl)hexanediamide with carrier solid base catalyst
A technology of solid base catalyst and adipamide, applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve problems such as increased steric hindrance, difficult experimental conditions, and difficult rearrangement , to achieve the effect of reducing the content, high activity and selectivity, and accelerating the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of 50% supported solid base catalyst
[0021] Take equal amounts of N,N,N',N'-tetrakis(β-hydroxypropyl)adipamide and sodium oxide, put them into a mortar, grind them, repeat 3 times, and then put them in an oven at 110°C Dry for 3 hours, take out, grind, and set aside.
Embodiment 2
[0023] Add 13.3g (0.10mol) of diisopropanolamine and 0.18g (accounting for 0.8% of the total material) of 50% NaOH / adipamide-loaded solid base, heated and stirred, heated to 145°C, and when the pressure was 0.05MPa, 8.7g (0.05mol) of dimethyl adipate was added dropwise, and the dripping was completed in 40 minutes. Stop the depressurization, and grind crushed solids.
[0024] Take out 2.3g without any treatment, directly dry to obtain 2.2g, yield 95.78%. The melting point is 115~122°C. Add 8 mL of absolute ethanol to the remaining 13.76 g, heat and stir until the solid becomes a fine slurry, stop the reaction, cool, filter with suction, and dry to obtain 12.5 g, yield 92.86%, melting point 118~121 °C.
Embodiment 3
[0026] Add 13.3g (0.10mol) of diisopropanolamine and 0.23g (accounting for 1.05% of the total material) of 50% NaOH / adipamide-loaded solid base, heated and stirred, heated to 145°C, and when the pressure was 0.05MPa, 8.7g (0.05mol) of dimethyl adipate was added dropwise, and the dripping was completed in 40 minutes. Stop the depressurization, and grind crushed solids. Add 10.2ml of absolute ethanol, heat, stir until completely dissolved, stop the reaction, cool, filter with suction, and dry to obtain the target product. The yield is 92.09%, and the melting point is 117~120°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com