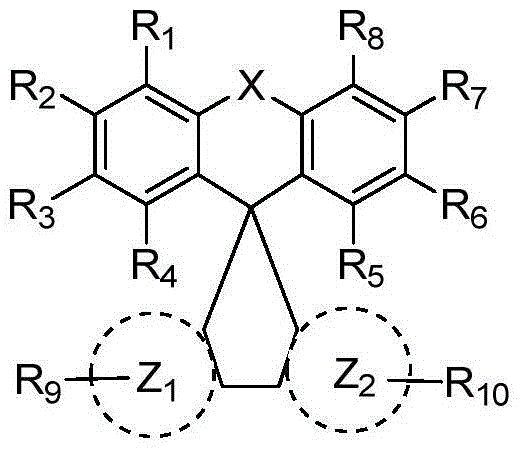
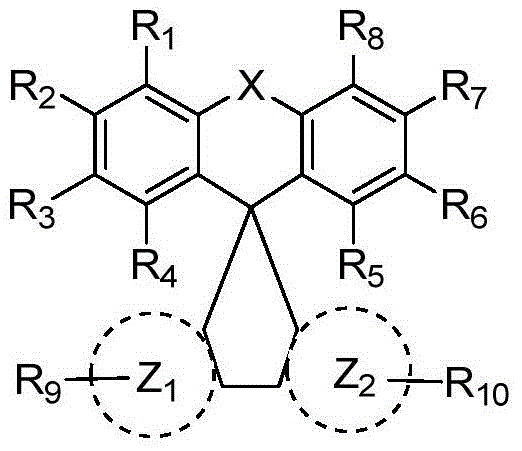
Organic luminescence compound and organic electroluminescence element containing organic luminescence compound
A technology of electroluminescent components and light-emitting compounds, which is applied in the fields of silicon organic compounds, electrical components, organic chemistry, etc., can solve the problems that the development has not been fully carried out, and achieve the effect of excellent power efficiency and excellent light-emitting characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0106] Synthesis Example 1: Synthesis of [Compound 1]
[0107] [Reaction Formula 1-1] Synthesis of [Intermediate 1-a]
[0108]
[0109] 1-naphthaleneboronic acid (12.5g, 0.073mol), 1-bromo-2-iodobenzene (17.3g, 0.061mol), tetrakis (triphenylphosphine) palladium (3.62g, 0.003mol), potassium carbonate (16.9g , 0.122mol) by adding 1,4-two 150 mL of alkanes, 150 mL of toluene, and 60 mL of distilled water were refluxed for 12 hours. Cool to room temperature, extract with ethyl acetate, and separate by column chromatography to obtain 14.7 g of (intermediate 1-a) (yield 85%).
[0110] [Reaction Formula 1-2] Synthesis of [Intermediate 1-b]
[0111]
[0112] [Intermediate 1-a] (3.5 g, 12.2 mmol) was added to 50 mL of tetrahydrofuran, and n-butyllithium (5.8 mL, 14.6 mmol) was added dropwise at -78°C, followed by stirring for about 1 hour. At the same temperature, a solution of 9-xanthone (2.1 g, 10.8 mmol) dissolved in 10 mL of tetrahydrofuran was slowly added dropwise, sti...
Synthetic example 2
[0122] Synthesis Example 2: Synthesis of [Compound 7]
[0123] Use 9-phenanthreneboronic acid instead of 1-naphthaleneboronic acid used in the above [Reaction Formula 1-1], use 2-bromo-1-iodonaphthalene instead of 1-bromo-2-iodobenzene, and 1-naphthaleneboronic acid instead 2-Naphthylboronic acid used in the above [Reaction Formula 1-5] was obtained by the same method as in Synthesis Example 1 to obtain [Compound 7] (66% yield).
Synthetic example 3
[0124] Synthesis Example 3: Synthesis of [Compound 14]
[0125] [Reaction Formula 3-1] Synthesis of [Intermediate 3-a]
[0126] Using quinoline-5-boronic acid instead of 1-naphthaleneboronic acid used in the above [Reaction Formula 1-1], and using 2,4-dibromo-1-iodobenzene instead of 1-bromo-2-iodobenzene, [Intermediate 3-a] was obtained by the same method as [Reaction Formula 1-1] to [Reaction Formula 1-3] (yield 88%).
[0127] [Reaction Formula 3-2] Synthesis of [Compound 14]
[0128] Use 2-naphthylboronic acid instead of 1-naphthylboronic acid used in the above [Reaction Formula 1-1], use [Intermediate 3-a] synthesized in the above [Reaction Formula 3-1] instead of 1-bromo- 2-iodobenzene, using the same method to obtain [compound 14] (yield 70%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com