Protein entrapping method
A protein and solution technology, which is applied in the field of protein loading, can solve the problems of drug waste, low drug release, uninspected particle size, and drug loading, and achieve fast release speed, high drug release, and low drug loading. The effect of high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
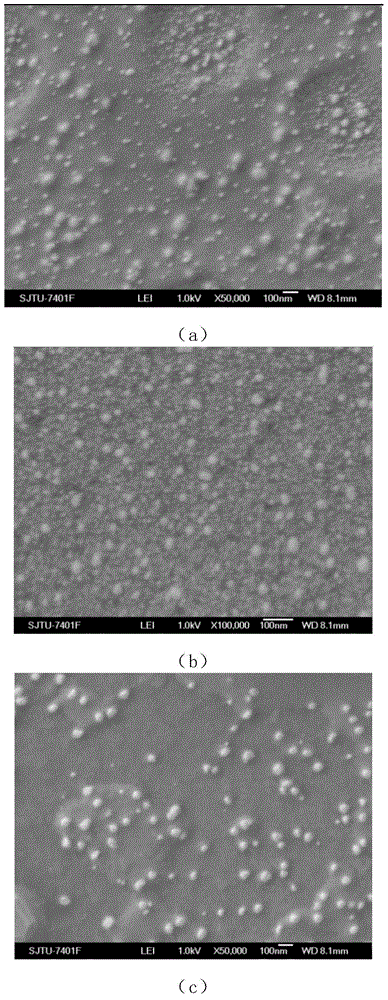
[0030] This example involves the preparation and particle size determination of blank nanoparticles. By controlling the concentration, ratio, and drop rate of the reactants, the conditions for forming nanoparticles are determined, and the subsequent exploration steps for preparing drug-loaded nanoparticles are reduced. The specific operation is as follows:
[0031] Step 1, dissolving chitosan quaternary ammonium salt (HACC) in ultrapure water to prepare an aqueous solution of chitosan quaternary ammonium salt with a mass concentration of 1.5-2.3 mg / mL.
[0032] Step 2: Add sodium tripolyphosphate (TPP) aqueous solution with a mass concentration of 0.35-0.6 mg / mL dropwise into the above solution at a rate of 0.2-0.8 mL / min through a constant-flow pump, and stir for 20-40 min after dropping. The volume ratio of sodium tripolyphosphate (TPP) solution to chitosan quaternary ammonium salt solution was controlled in the range of 1:(1~2).
[0033] Step 3, using a high-speed centrifu...
Embodiment 2
[0042] This embodiment relates to the preparation and particle size determination of BSA-loaded nanoparticles, and the specific operations are as follows:
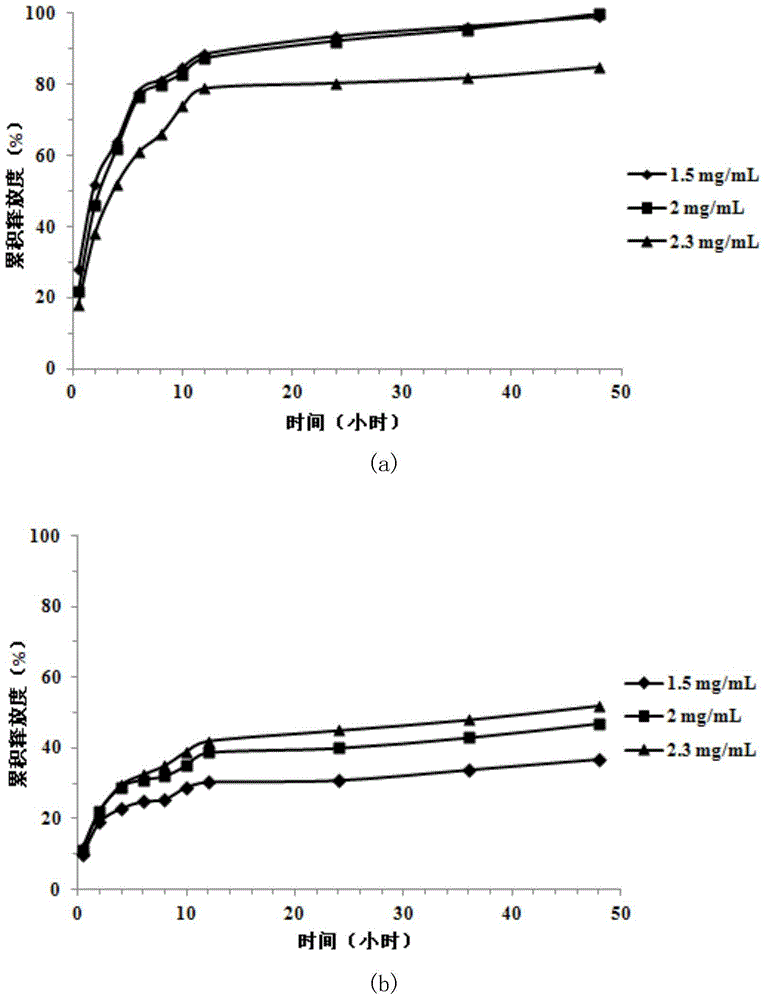
[0043] Step 1, bovine serum albumin (BSA) is dissolved in the chitosan quaternary ammonium salt (HACC) aqueous solution of 2.0mg / mL with different mass concentration 0.5,0.9,1.3mg / mL;
[0044] Step 2: Add 0.45mg / mL sodium tripolyphosphate (TPP) aqueous solution dropwise to the above solution at a rate of 0.2-0.8mL / min through a constant flow pump, and stir for 20-40min after dropping. The volume ratio of sodium tripolyphosphate (TPP) solution to chitosan quaternary ammonium salt solution was controlled in the range of 1:(1~2).
[0045] Step 3, using a high-speed centrifuge to centrifuge at 4500-5000 rpm for 12-18 minutes. Take the supernatant and filter it with a 0.22 μm membrane filter.
[0046] 1 mL of the prepared nanoparticles was directly absorbed, and the particle size was measured by a dynamic light scattering ins...
Embodiment 3
[0050] This embodiment relates to the preparation of loaded rHAm nanoparticles, the specific operations are as follows:
[0051] Step 1, dissolving recombinant amelogenin (rHAm) in 1.5-2.3 mg / mL chitosan quaternary ammonium salt (HACC) aqueous solution at a mass concentration of 0.5 mg / mL;
[0052] Step 2: Add 0.35-0.6 mg / mL sodium tripolyphosphate (TPP) aqueous solution dropwise to the above solution through a constant flow pump at a rate of 0.2-0.8 mL / min, and stir for 20-40 min after the dropping is completed. The volume ratio of sodium tripolyphosphate (TPP) solution to chitosan quaternary ammonium salt solution was controlled in the range of 1:(1~2).
[0053] Step 3, using a high-speed centrifuge to centrifuge at 4500-5000 rpm for 12-18 minutes. The supernatant was taken and filtered with a 0.22 μm filter membrane to obtain rHAm-loaded nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com