Mixed oxide catalyst for preparing highalcohol with synthesis gas
A mixed oxide and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, preparation of hydroxyl compounds, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
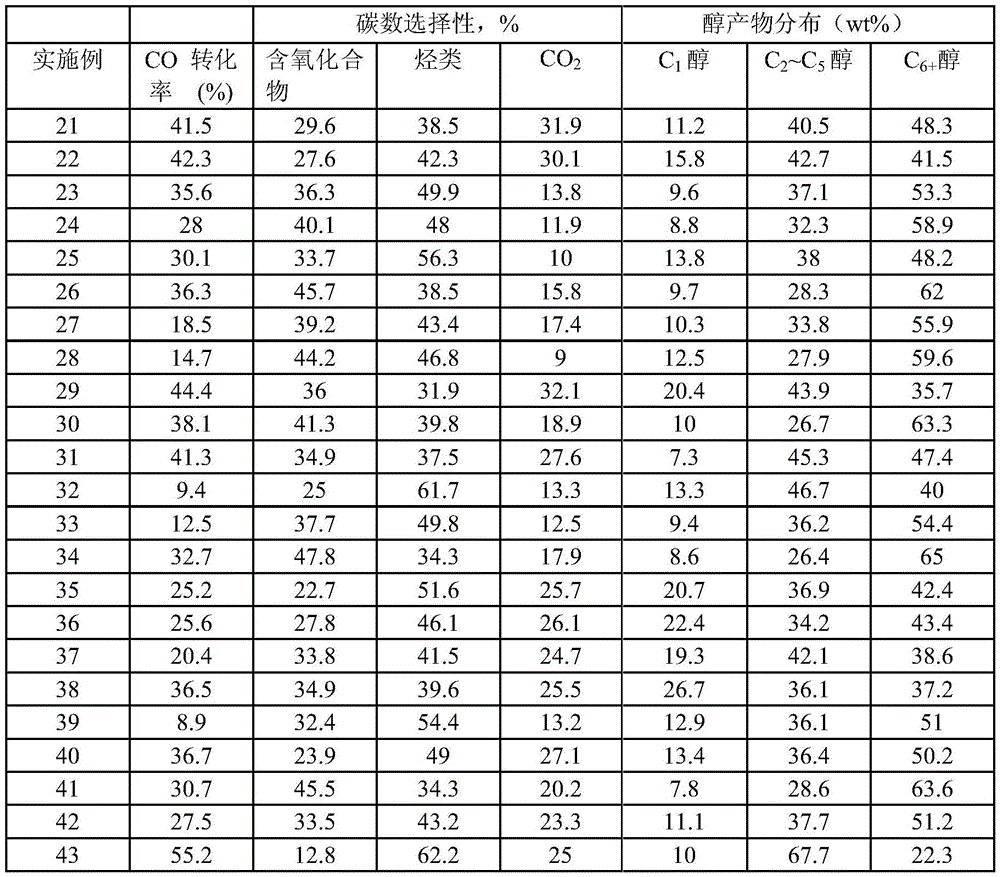
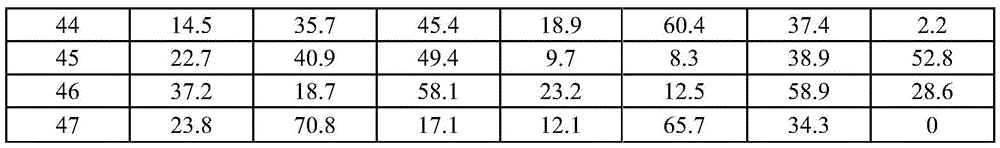
Examples
Embodiment 1
[0066] [Example 1] Preparation of Catalyst A1
[0067] Zn(NO 3 ) 2 ·6H 2 O aqueous solution and Co(NO 3 ) 2 ·6H 2O is dissolved in a certain amount of deionized water according to the molar ratio Co / Zn=2 / 1 to form a mixed solution with a total metal concentration of 0.1mol / L, and then dissolve sodium carbonate in a certain amount of deionized water to form a concentration of 0.1 mol / L lye. Add the mother liquor, namely water, into the beaker, adjust the titration temperature to 65° C., control the titration pH=7, and co-precipitate the above two solutions in the stirred mother liquor in a co-current manner. After the titration, it was aged at 65°C for 8 hours, centrifuged and washed 6 times, dried in an oven at 80°C for 24 hours, and then transferred to a muffle furnace for 6 hours at 300°C. Catalyst A1 is obtained after calcination.
Embodiment 2
[0068] [Example 2] Preparation of Catalyst A2
[0069] 50%Mn(NO 3 ) 2 Aqueous solution and Co(NO 3 ) 2 ·6H 2 O is dissolved in a certain amount of deionized water according to the molar ratio Co / Mn=2 / 1 to form a mixed solution with a total metal concentration of 0.5mol / L, and then dissolve potassium carbonate in a certain amount of deionized water to form a concentration of 1mol / L lye. Add the mother liquor, ie water, into the beaker, adjust the titration temperature to 20° C., control the titration pH=8, and co-precipitate the above two solutions in the stirred mother liquor in a co-current manner. After the titration, it was aged at 20°C for 4h, centrifuged and washed 6 times, dried in an oven at 100°C for 12h, and then transferred to a muffle furnace for 4h roasting at 350°C. Catalyst A2 is obtained after calcination.
Embodiment 3
[0070] [Example 3] Preparation of Catalyst A3
[0071] Will Co(NO 3 ) 2 ·6H 2 O and Fe(NO 3 ) 3 9H 2 O is dissolved in a certain amount of deionized water according to the molar ratio Co / Fe=4 / 1 to form a mixed solution with a total metal concentration of 1mol / L, and then dissolve sodium carbonate in a certain amount of deionized water to form a concentration of 1mol / L L lye. Add the mother liquor into the beaker, adjust the titration temperature to 40° C., control the titration pH=10, and co-precipitate the above two solutions in the stirred mother liquor in a co-current manner. After the titration, it was aged at 40°C for 10 hours, centrifuged and washed for 6 times, dried in an oven at 120°C for 12 hours, and then transferred to a muffle furnace for 8 hours at 250°C. Catalyst A3 is obtained after calcination.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com