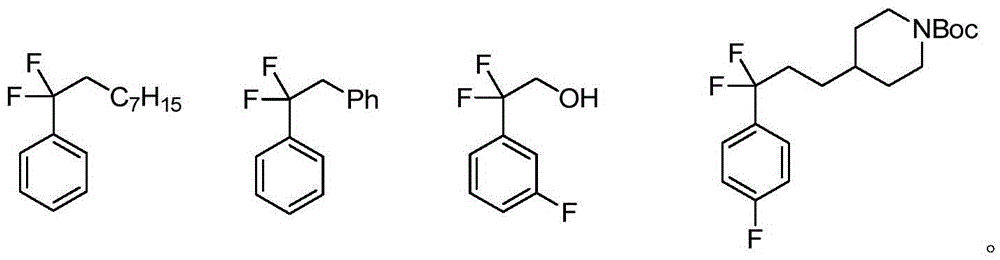
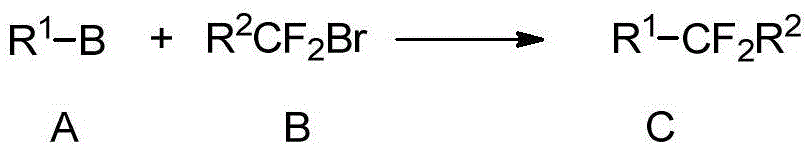Difluoroalkyl-substituted aryl or hetetoaryl compounds, and preparation method and application thereof
A difluoroalkyl and compound technology, applied in the preparation of carbon-based compounds, the preparation of hydroxyl compounds, the preparation of organic compounds, etc., can solve the problems of poor functional group compatibility, harsh reaction conditions, and poor broad spectrum.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108]
[0109] Into a 25mL reaction tube, add 110mg (0.9mmol) phenylboronic acid, 6.6mg (5mol%, refers to the percentage of 5-bromo-5,5'-difluoropentylbenzene molar weight) NiCl 2 DME, 8mg (5mol%) 4,4'-d t Bubpy (4,4'-di-tert-butylbipyridine), 14.6mg (20mol%) DMAP, 166mg (1.2mmol) K 2 CO 3 , 4 mL of triglyme, injected with 157 mg (0.6 mmol) of 5-bromo-5,5'-difluoropentylbenzene, and stirred at 80°C for 24 hours, the isolated yield was 92%. The purity is greater than 95% identified by hydrogen spectrum. 1 HNMR (400MHz, CDCl 3 )δ7.48–7.38(m,5H),7.27(t,J=7.3Hz,2H),7.20–7.11(m,3H),2.58(t,J=7.6Hz,2H),2.21–2.07(m ,2H),1.68–1.59(m,2H),1.54–1.45(m,2H). 19 FNMR (376MHz, CDCl 3 )δ-95.5(t, J=16.2Hz, 2F). 13 CNMR (125.7MHz, CDCl 3 )δ142.1, 137.5(t, J=26.6Hz), 129.5(t, J=1.5Hz), 128.34, 128.32, 128.30, 125.8, 124.9(t, J=6.2Hz), 123.0(t, J=242.1Hz) ,38.9(t,J=27.5Hz),35.7,31.1,22.2(t,J=4.0Hz).IR(thinfilm)ν max 3027,2934,1496,1452,1327cm -1 .MS(EI):m / z(%)260(M + ),240,127,91(1...
Embodiment 2
[0111]
[0112] Into a 25mL reaction tube, add 178.2mg (0.9mmol) 4-phenylphenylboronic acid, 6.6mg (5mol%, refers to the percentage of moles of 5-bromo-5,5'-difluoropentylbenzene) NiCl 2 DME, 8mg (5mol%) 4,4'-d t Bubpy (4,4'-di-tert-butylbipyridine), 14.6mg (20mol%) DMAP, 166mg (1.2mmol) K 2 CO 3 , 4 mL of triglyme, injected with 157 mg (0.6 mmol) of 5-bromo-5,5'-difluoropentylbenzene, and stirred at 80°C for 24 hours, the isolated yield was 95%. The purity is greater than 95% identified by hydrogen spectrum. 1 HNMR (400MHz, CDCl 3 )δ7.64(d, J=8.2Hz, 2H), 7.61(d, J=7.4Hz, 2H), 7.53(d, J=8.2Hz, 2H), 7.47(t, J=7.5Hz, 2H) ,7.38(t,J=7.4Hz,1H),7.27(t,J=7.4Hz,2H),7.21–7.11(m,3H),2.61(t,J=7.8Hz,2H),2.26–2.12( m,2H),1.73–1.61(m,2H),1.56–1.50(m,2H). 19 FNMR (376MHz, CDCl 3)δ-95.1(t, J=16.2Hz, 2F). 13 CNMR (125.7MHz, CDCl 3 )δ142.4(t, J=1.4Hz), 142.1, 140.2, 136.3(t, J=26.9Hz), 128.8, 128.30, 128.27, 127.7, 127.12, 127.05, 125.7, 125.4(t, J=6.1Hz) ,123.1(t,J=242.1Hz),38.8(t...
Embodiment 3
[0114]
[0115] Into a 25mL reaction tube, add 178.2mg (0.9mmol) of 3-phenylphenylboronic acid, 6.6mg (5mol%, refers to the percentage of moles of 5-bromo-5,5'-difluoropentylbenzene) NiCl 2 DME, 8mg (5mol%) 4,4'-d t Bubpy (4,4'-di-tert-butylbipyridine), 14.6mg (20mol%) DMAP, 166mg (1.2mmol) K 2 CO 3 , 4 mL of triglyme, injected with 157 mg (0.6 mmol) of 5-bromo-5,5'-difluoropentylbenzene, and stirred at 80°C for 24 hours, the isolated yield was 90%. The purity is greater than 95% identified by hydrogen spectrum. 1 HNMR (400MHz, CDCl 3 )δ7.71(s,1H),7.67(d,J=7.8Hz,1H),7.63(d,J=7.8Hz,2H),7.54–7.44(m,4H),7.41(t,J=7.3 Hz,1H),7.28(t,J=7.0Hz,2H),7.22–7.14(m,3H),2.63(t,J=7.6Hz,2H),2.30–2.14(m,2H),1.74–1.64 (m,2H),1.61–1.51(m,2H). 19 FNMR (376MHz, CDCl 3 )δ-95.4(t, J=16.3Hz, 2F). 13 CNMR (125.7MHz, CDCl 3 )δ142.1, 141.5, 140.5, 138.0 (t, J = 26.8Hz), 128.9, 128.32, 128.29, 127.7, 127.2, 125.8, 123.78 (t, J = 6.2Hz), 123.72 (t, J = 6.3Hz), 123.0 (t, J=242.4Hz), 39.0(t, J=27...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com