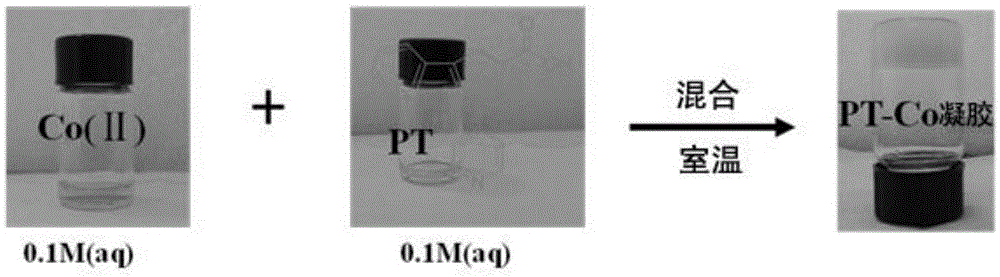
Gelator and preparation method thereof as well as supramolecular metal hydrogel using gelator and preparation method and application of supramolecular metal hydrogel
A gel factor and hydrogel technology, applied in the field of supramolecular metal hydrogel and its preparation, can solve problems such as limited application and achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: the preparation of metal hydrogel and Co 2+ Specific recognition experiment
[0046] (1) Synthesis of gelatin factor (PT)
[0047] Slowly add 10 mL of methanol solution containing 0.53 g of 4-pyridineformaldehyde to 15 mL of aqueous solution containing 1 g of tryptophan (L-tryptophan or D-tryptophan) and 0.28 g of potassium hydroxide at room temperature, and stir for 2 hours , to obtain intermediate product X or intermediate product Y; after the reaction was completed, the reaction system was placed in an ice bath for cooling, then 0.21 g of sodium borohydride was added, and reacted for 3 hours; the addition of 50% acetic acid was neutralized to a pH of 4~5 Continue to stir and react for 2 hours to obtain the crude product of gelatin factor; filter the crude product, wash twice with water and methanol successively, and recrystallize with deionized water to obtain 1.2g white pure gelatin factor (L - gelling factor or D-gelling factor).
[0048] Its react...
Embodiment 2
[0058] Embodiment 2: the preparation of supramolecular metal hydrogel
[0059] (1) Synthesis of gelling factor
[0060] Slowly add 10 mL of methanol solution containing 0.53 g of 4-pyridineformaldehyde to 15 mL of aqueous solution containing 1 g of tryptophan (L-tryptophan or D-tryptophan) and 0.28 g of potassium hydroxide at room temperature, and stir for 2 hours After the reaction was completed, the reaction system was placed in an ice bath for cooling, then 0.21g of sodium borohydride was added for reaction for 3 hours; the addition of 50% acetic acid was neutralized to pH 4 to 5 and the stirring reaction was continued for 2 hours. The crude product of gelatin factor was obtained; the crude product was filtered, washed twice with water and methanol respectively, and then recrystallized with deionized water to obtain 1.2 g of white pure gelatin factor.
[0061] (2) Preparation of metal hydrogels
[0062] Weigh 29.5 mg of the gelatin factor (L-gelatin factor or D-gelatin fa...
Embodiment 3
[0063] Embodiment 3: Sustained release experiment
[0064] Take by weighing 29.5mg of gel factor (L-gel factor or D-gel factor) sample, prepare 1mL0.1mol / L gel factor aqueous solution, the solution is colorless and transparent; the above gel factor aqueous solution and divalent cobalt ion The aqueous solution is mixed in a glass bottle according to the molar ratio of gel factor and divalent cobalt ion 2:1, adjust the pH to 7~8, add 5uL 10mmol / L methylene blue (MB) aqueous solution, shake, and the methylene blue The addition of Co has no obvious effect on the gelling factor-Co metal hydrogel, only the color changed from pink to blue.
[0065]Add 2 mL of deionized water on top of the metal hydrogel with added methylene blue. As time goes by, the water above turns from colorless to light blue. Take out a small amount of water above the gel at intervals, dilute to 2mL, and conduct UV test experiments. It was found that the characteristic absorption peak of methylene blue appear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com