Gel polymer electrolyte with self-crosslinking characteristic for lithium ion battery
A gel polymer and lithium-ion battery technology, applied in the direction of non-aqueous electrolyte battery, electrolyte battery manufacturing, electrolyte immobilization/gelation, etc., can solve implementation difficulties, high γ-ray radiation human body damage, gel electrolyte issues such as adverse effects on stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
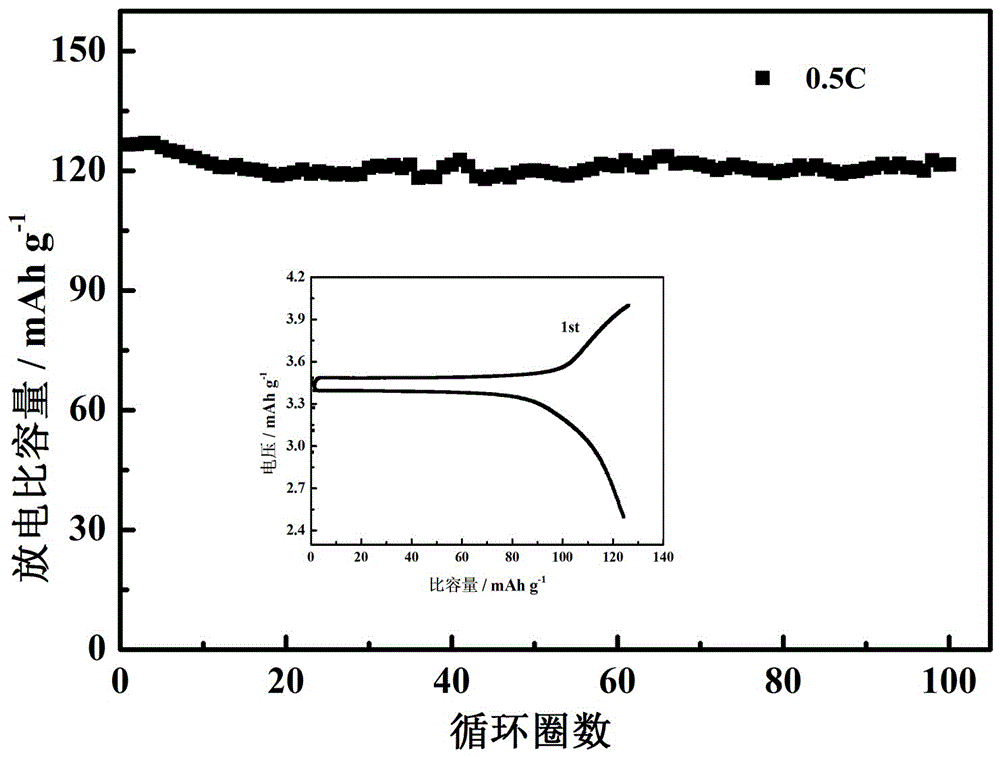
[0029] Dissolve 1.32g of lithium bisoxalate borate (LiBOB) into 5ml of propylene carbonate (PC) to dissolve completely. At room temperature, add 2g of silylmethoxy-terminated polypropylene oxide to the electrolyte solution of lithium bisoxalate borate and propylene carbonate and stir evenly (indicate the viscosity number average molecular weight), and then place on a polytetrafluoroethylene plate, With the cellulose membrane as the supporting framework, the uniformly stirred polymer electrolyte was scraped and coated on both sides of the cellulose membrane, and then heated in an oven at 45°C for 20 hours, the polymer electrolyte solidified and formed a film by itself. The mass fraction of the cross-linked polyether in the gel electrolyte after curing into a film is 25% (excluding the cellulose film).
Embodiment 2
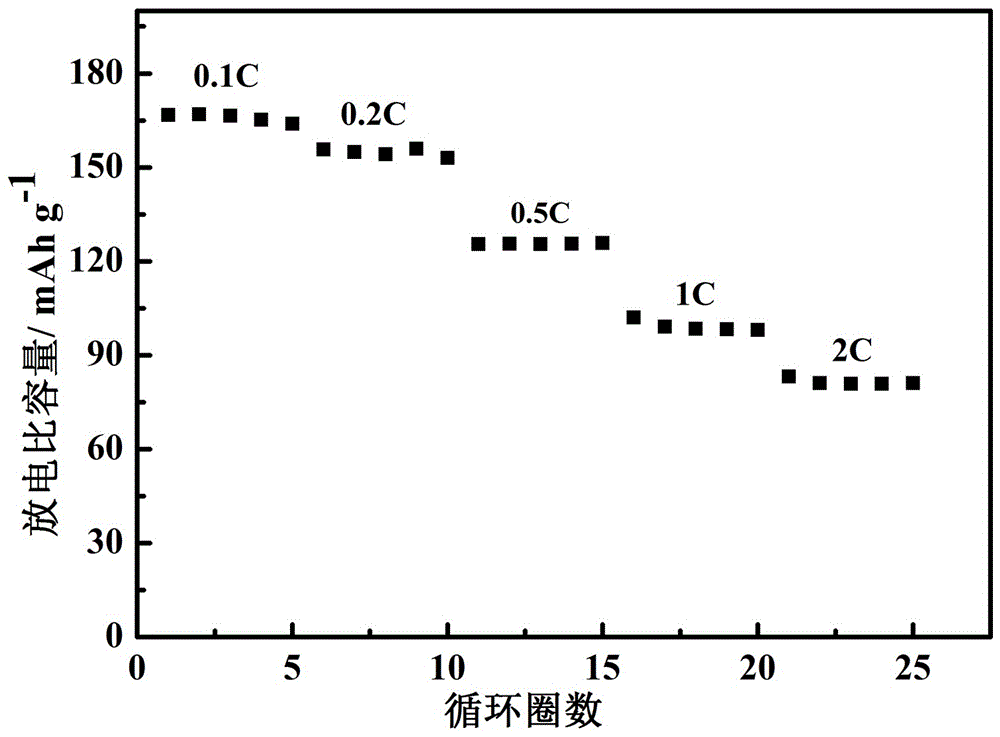
[0031] Dissolve 0.88 g of lithium bis(salicylate) borate (LBSB) into 5 ml of propylene carbonate (PC) and ethylene carbonate (EC) (v:v = 1:1) to dissolve completely. At room temperature, add 2g of silicon methoxy-terminated polyethylene oxide to the electrolyte solution of lithium bisoxalate borate and propylene carbonate and stir evenly, and then place it on a polytetrafluoroethylene plate with a cellulose membrane as the supporting framework , the uniformly stirred polymer electrolyte was scraped onto both sides of the cellulose membrane, and then heated in an oven at 45°C for 18 hours, the polymer electrolyte solidified into a film by itself. The mass fraction of the cross-linked polyether in the gel electrolyte is 27% (except for the cellulose film).
Embodiment 3
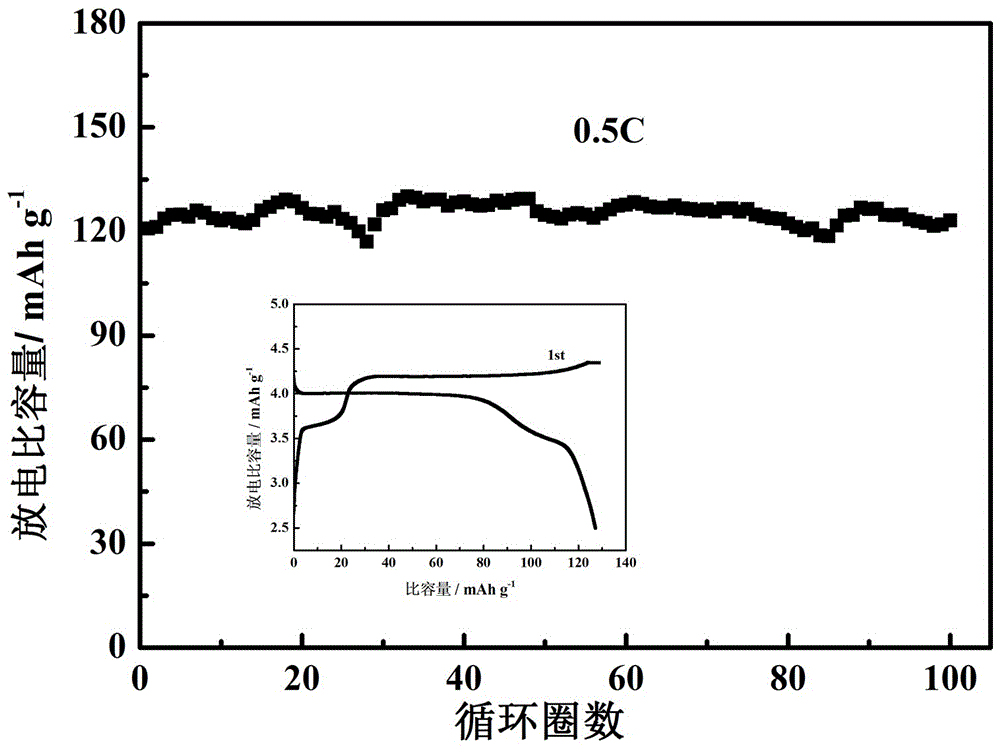
[0033] Dissolve 1.12 g of lithium bismalonate borate (LiBMB) into 5 ml of propylene carbonate (PC) and ethylene carbonate (EC) (v:v = 1:1) to dissolve completely. At room temperature, add 3 g of silicon methoxy-terminated polyethylene oxide to the electrolyte solution of lithium bisoxalate borate and propylene carbonate and stir evenly, and then place it on a polytetrafluoroethylene plate with a polyimide porous membrane In order to support the skeleton, the uniformly stirred polymer electrolyte was scraped and coated on both sides of the polyimide film, and then heated in an oven at 45°C for 10 hours, the polymer electrolyte cured itself to form a film. The mass fraction of the cross-linked polyether in the gel electrolyte after curing to form a film is 38% (except for the polyimide film).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com