Atypical hemolytic uremic syndrome (ahus) biomarker proteins
A biomarker, hemolytic technology, applied in the direction of anti-animal/human immunoglobulin, biological test, immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0282] Example 1. Materials and Methods
[0283] urine sample
[0284] Mix freshly collected urine immediately with protease inhibitors. Concentrations of several analytes including NGAL, cystatin C, clusterin, TIMP-1, β2-microglobulin, C5b9, C5a, and creatinine.
[0285] NGAL levels were measured in urine with a commercially available kit (R&D Systems, Minneapolis, MN; product number: DLCN20). Briefly, urine samples were diluted 1:3 with the calibration diluent RD5-24 provided in the kit. Add 50 μL of each sample or kit standard control (NSO-expressed recombinant human lipocalin-2) to the wells of the assay plate in duplicate, each well containing 100 μL of the assay diluent RD1- 52. After two hours of incubation at 4°C in the refrigerator, the wells were washed 4 times with 200 μL of wash solution per well. 200 μL of anti-NGAL conjugate labeled with an enzyme (horseradish peroxidase) was added to each well and incubated in the refrigerator at 4°C for two hours. The...
Embodiment 2
[0307] Example 2: Results
[0308] ongoing complement activation markers
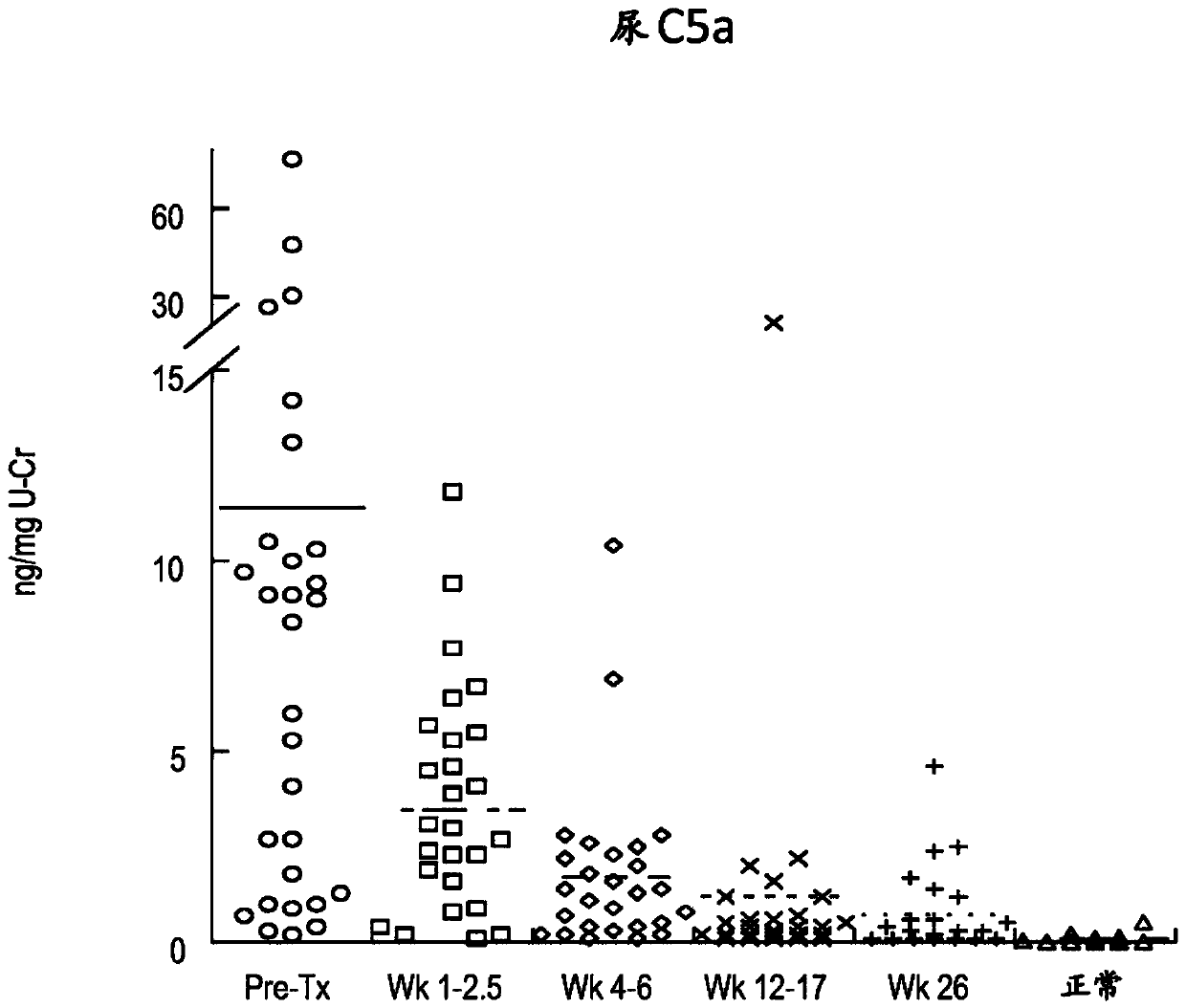
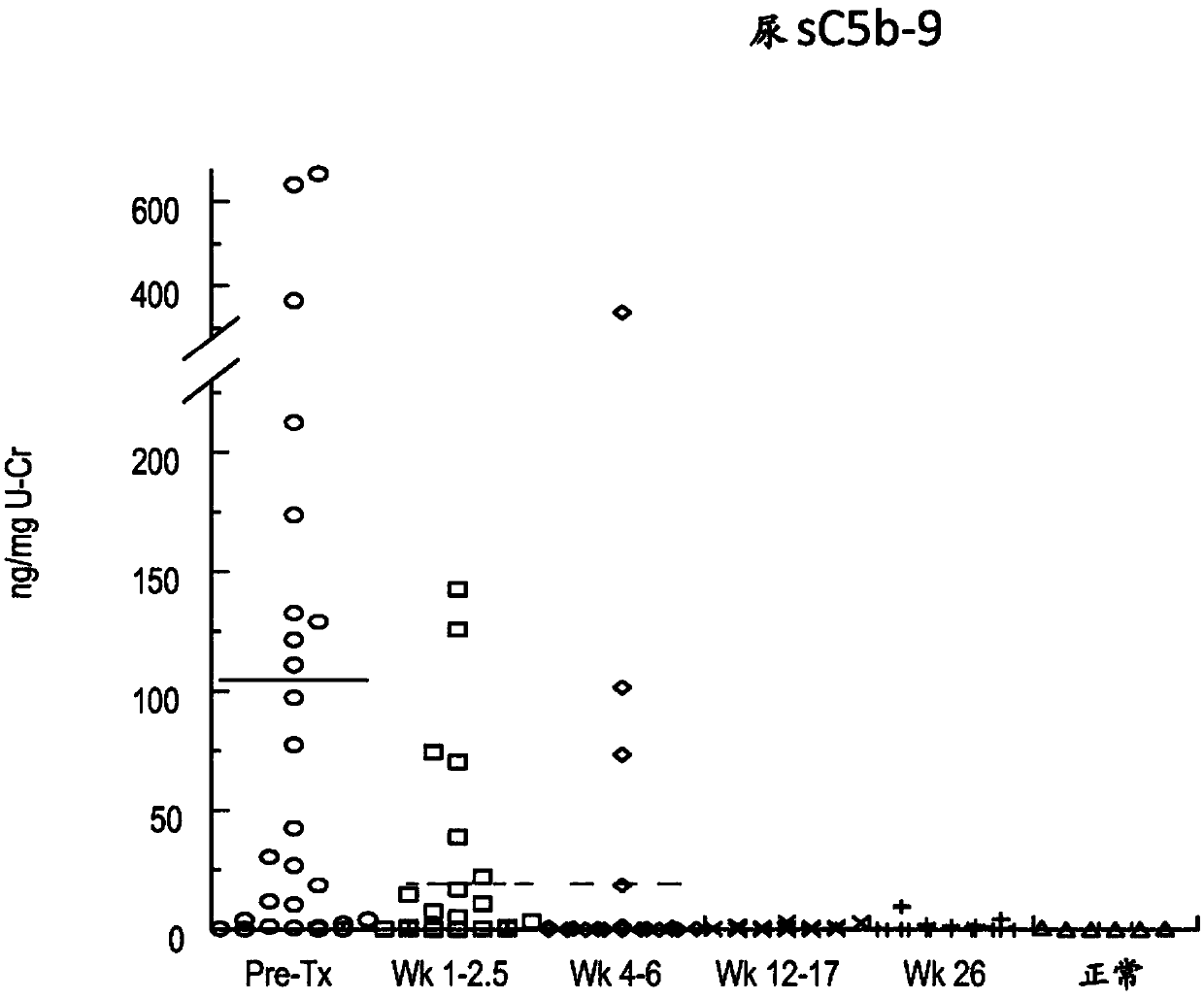
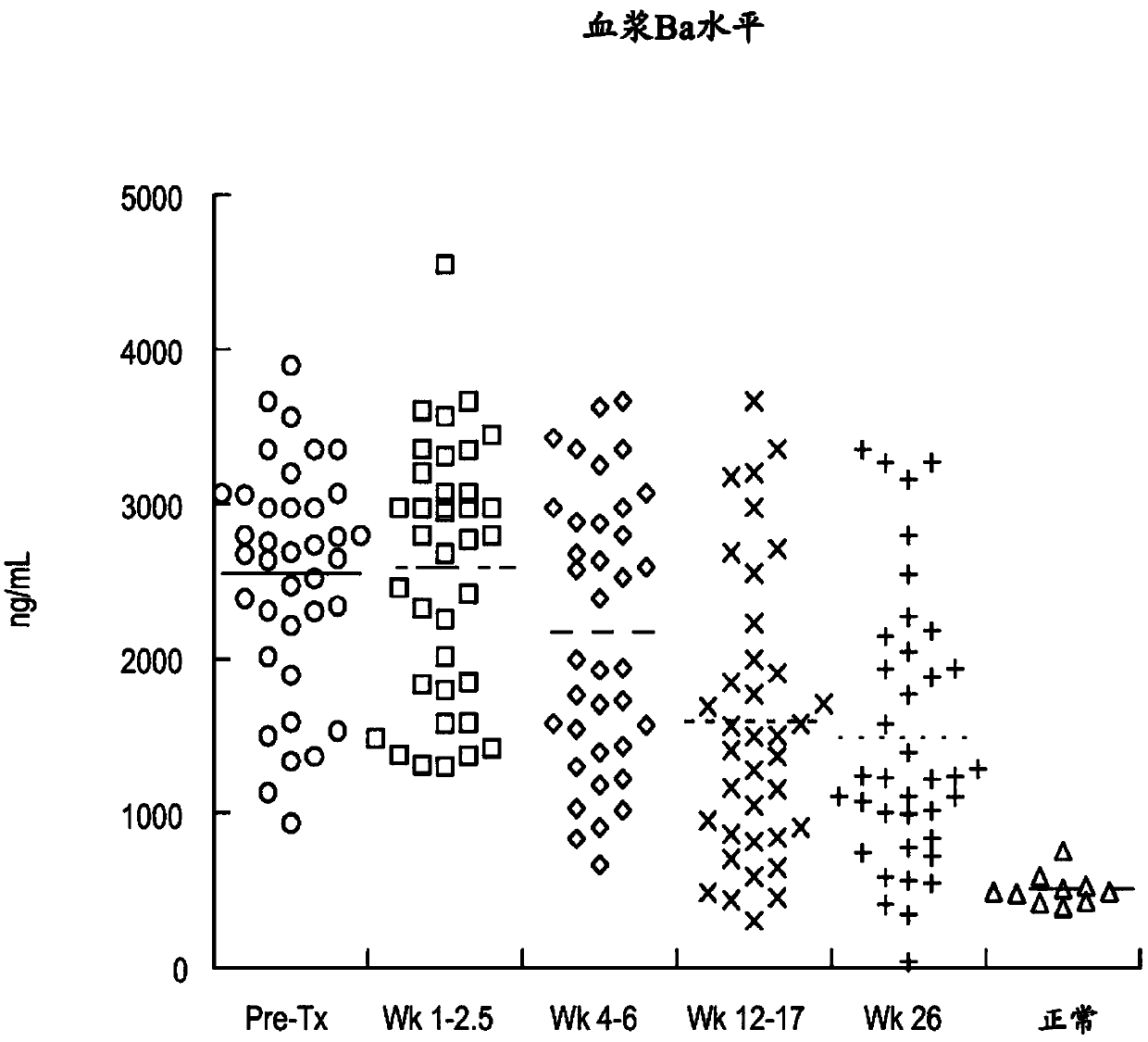
[0309] As summarized in Table 2 below, plasma concentrations of the complement components Ba and sC5b9 and urine concentrations of C5a and sC5b-9 were elevated in most aHUS patients relative to concentrations in biological fluid samples from healthy volunteers. see also Figures 1A-1F .
[0310] Table 2
[0311] aHUS biomarker protein
Increased n / N(%) from baseline
P-value
Plasma Ba
35 / 35(100.0)
<0.0001
plasma sC5b-9
37 / 38(97.4)
<0.0001
Urine C5a
26 / 29(89.7)
0.0007
urine sC5b-9
23 / 27(85.2)
0.0025
[0312] *"N" indicates the total number of patients for which a given biomarker was assessed, while "n" indicates the number of those "N" patients with elevated biomarker protein levels.
[0313] These results suggest a significant ongoing systemic alternative pathway complement activation in aHUS patients.
[0314] The mean ...
Embodiment 3
[0387] Example 3: Baseline levels of selected aHUS biomarker proteins in aHUS patients
[0388] At baseline, before eculizumab treatment, significant complement activation, vascular inflammation / injury, and parenchymal signs of organ damage were observed in aHUS patients, regardless of use of plasmapheresis / transfusion or platelet count, Hp or Normal laboratory value of LDH. As demonstrated by the data shown in Table 11, the concentrations of aHUS biomarkers of complement activity, vascular inflammation, endothelial activation and injury, coagulation, and renal injury were significantly elevated in aHUS patients compared to healthy subjects of.
[0389] Table 11
[0390]
[0391]
[0392] NHV means the normal human value or concentration of a given aHUS biomarker protein reported in the table.
[0393] "creat" means creatinine, the concentration of which was used to normalize some of the biomarker concentrations reported in the table.
[0394] CAP means alternative...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com