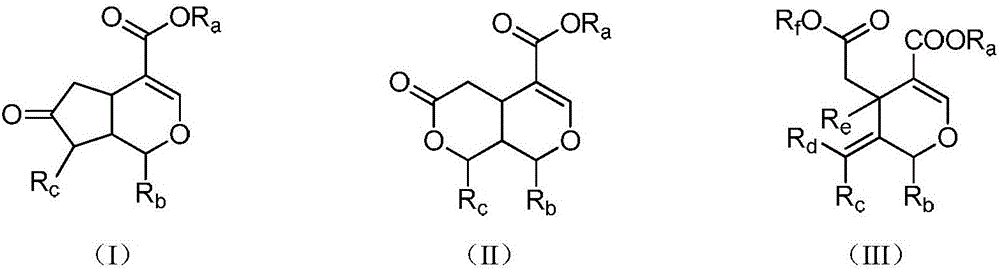
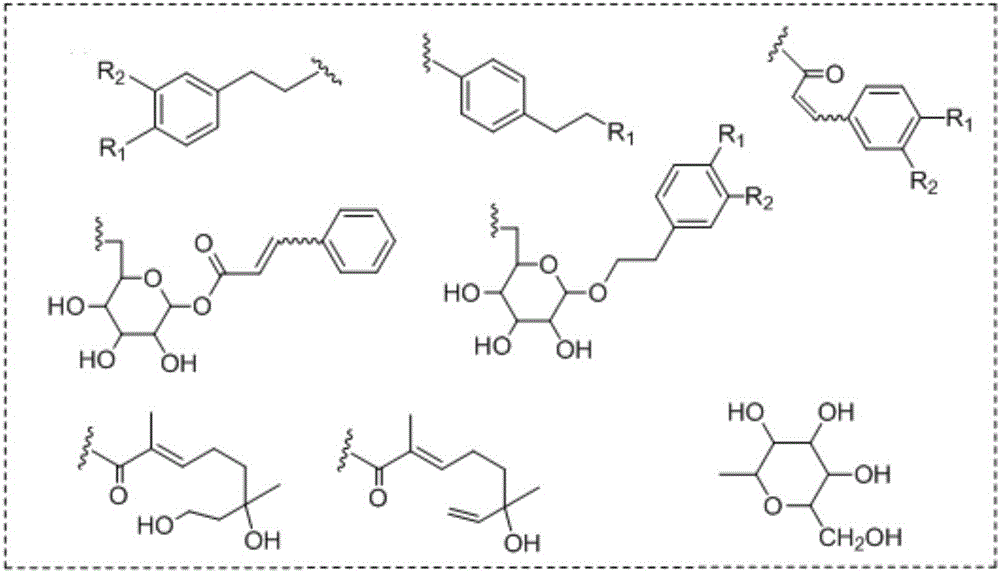
Iridoid compound and application of metabolite of iridoids compound in preparing drug-resistance bacterium infection resisting drug
A technology of iridoids and metabolites, which is applied to the active ingredients of hydroxyl compounds, antibacterial drugs, drug combinations, etc., and can solve the problems of antibiotics being difficult to work and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0026] Experimental Example 1: Separation and structure identification of in vivo metabolites of syringpicroside A
[0027] Syringin A was ultrasonically dissolved in drinking water, administered orally to 20 male SD rats at a dose of 100 mg / kg, and the urine was collected for a total of 20 days. A total of 2.4 L of urine was collected and stored in a freezer. The collected urine was extracted four times with ethyl acetate and n-butanol respectively. The ethyl acetate layer extract and the n-butanol layer extract were concentrated respectively, and the endogenous substances were tracked in parallel with blank urine through various column chromatography methods, and the in vivo metabolites of syringpicroside A in urine were analyzed. Separation and purification, a total of 9 metabolites were isolated, the metabolic pathway is as follows.
[0028]
Embodiment 2
[0029] Embodiment 2: The determination of the minimum inhibitory concentration (MIC) of iridoids and metabolites to 4 kinds of bacteria
[0030] Tested strains: Staphylococcus aureus (MSSA)
[0031] Methicillin-resistant Staphylococcus aureus (MRSA)
[0032] Penicillin-resistant Streptococcus pneumoniae (PRSP)
[0033] Vancomycin-resistant enterococci (VRE) were clinically isolated from the Laboratory Department of the 463rd Hospital of the People's Liberation Army.
[0034] Drugs and reagents: vancomycin hydrochloride for injection (VH, Eli Lilly Japan K.K); normal saline (Shandong Yuwang Industrial Co., Ltd.). Beef extract; peptone; NaCl; NaOH (Tianjin Bodi Chemical Co., Ltd.); water (sterile water).
[0035] Medium: Mueller-Hinton (MH) Broth Medium (g / L):
[0036]
[0037] Accurately weigh various components, stir and mix well, then add NaOH to adjust the pH to 7.2-7.4. Sterilize under high pressure steam at 0.1MPa for 30min.
[0038] Test compounds: Syringopicrosi...
experiment example 3
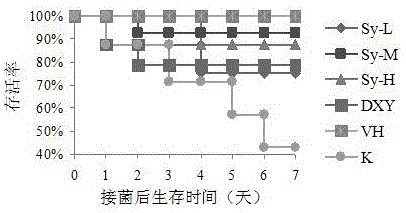
[0046] Experimental example 3: Determination of in vivo anti-Staphylococcus aureus infection activity of syringpicroside A (compound 1)
[0047]Select 72 Kunming mice, randomly divide them into 9 groups, 8 in each group, and mark them as blank group (K), positive group (VH), syringpicroside A high-dose group (Sy-H), syringpicroside A Medium dose group (Sy-M), syringpicroside A low dose group (Sy-L) and clove leaf group (DXY). After intragastric administration, methicillin-resistant Staphylococcus aureus (10 9 CFU / ml) 2 times, 0.1ml / 10g for the first time, 0.15ml / 10g for the second time. The administration was continued, and the number of deaths in each group was observed after 7 days.
[0048] Grouping and dosing are as follows:
[0049] Blank group: normal saline
[0050] VH group (positive group): vancomycin hydrochloride (300mg / kg, clinical dose)
[0051] DXY group (clove leaf group): clove leaf (82.2mg / kg, calculated as tablet core)
[0052] Sy-L group (low dose grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com