Lipidosome-polymer particle preparation of topical anaesthetic and preparation method of lipidosome-polymer particle preparation
A technology of local anesthetics and liposomes, applied in the field of liposome-polymer particle preparations of local anesthetics and its preparation, can solve the problems of high equipment requirements, increased production risks, dose dumping, etc., and achieve simple preparation methods , Good controlled release effect, good sustained release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
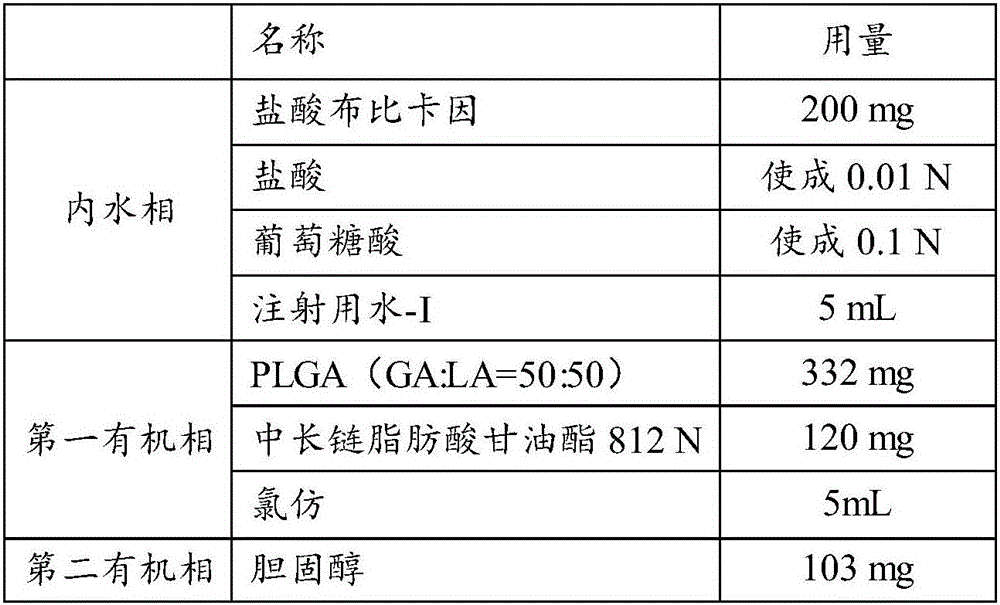
[0053] The prescription is shown in Table 1:
[0054]
[0055]
[0056] Carry out the following operations according to the formula in Table 1:
[0057] 1. Preparation of the inner aqueous phase: Disperse the prescribed amount of active drug (bupivacaine hydrochloride) and osmotic pressure regulator into the aqueous solution for injection whose pH is adjusted to 1.0 with hydrochloric acid, and stir until the materials are evenly dispersed in the inner aqueous phase solution, get the inner water phase;
[0058] 2. Preparation of the first organic phase: dissolving the polylactic acid-hydroxylactic acid copolymer of the prescribed amount in chloroform of a certain quality to obtain the first organic phase;
[0059] 3. Preparation of the second organic phase: respectively dissolving hydrogenated phospholipids and cholesterol in a certain amount of chloroform to obtain the second organic phase;
[0060] 4. Preparation of the external aqueous phase: dissolve the osmotic pre...
Embodiment 2
[0066] The prescription is shown in Table 2:
[0067]
[0068] Carry out the following operations according to the formula in Table 1:
[0069] 1. Preparation of the inner aqueous phase: Disperse the prescribed amount of active drug (bupivacaine hydrochloride) and osmotic pressure regulator into the aqueous solution for injection whose pH is adjusted to 1.0 with hydrochloric acid, and stir until the materials are evenly dispersed in the inner aqueous phase solution, get the inner water phase;
[0070] 2. Preparation of the first organic phase: dissolving the polylactic acid-hydroxylactic acid copolymer of the prescribed amount in chloroform of a certain quality to obtain the first organic phase;
[0071] 3. Preparation of the second organic phase: respectively dissolving hydrogenated phospholipids and cholesterol in a certain amount of chloroform to obtain the second organic phase;
[0072] 4. Preparation of the external aqueous phase: dissolve the osmotic pressure regula...
Embodiment 3
[0078] The prescription is shown in Table 3:
[0079]
[0080] Carry out the following operations according to the formula in Table 1:
[0081] 1. Preparation of the inner aqueous phase: Disperse the prescribed amount of active drug (bupivacaine hydrochloride) and osmotic pressure regulator into the aqueous solution for injection whose pH is adjusted to 1.0 with hydrochloric acid, and stir until the materials are evenly dispersed in the inner aqueous phase solution, get the inner water phase;
[0082] 2. Preparation of the first organic phase: dissolving the polylactic acid-hydroxylactic acid copolymer of the prescribed amount in chloroform of a certain quality to obtain the first organic phase;
[0083] 3. Preparation of the second organic phase: respectively dissolving hydrogenated phospholipids and cholesterol in a certain amount of chloroform to obtain the second organic phase;
[0084] 4. Preparation of the external aqueous phase: dissolve the osmotic pressure regula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com