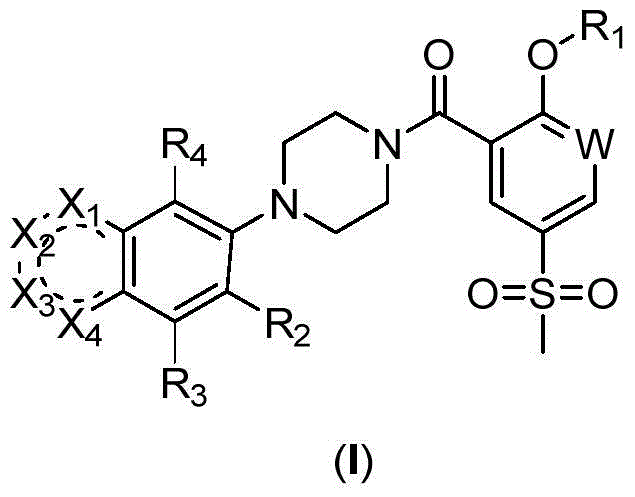
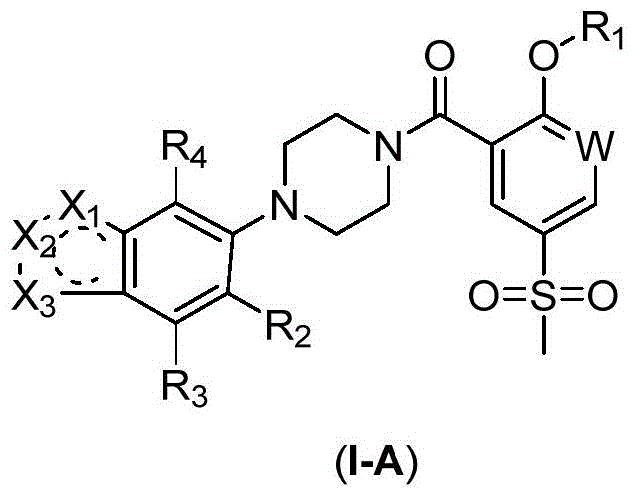
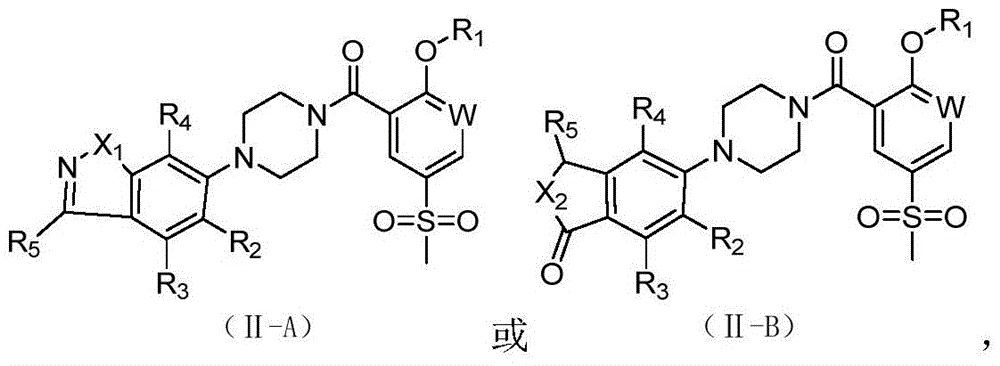
2-Substituted-oxy-5-methylsulfonyl aryl piperazine acidamide analogue and preparation method and application thereof
A technology of aryl piperazine amides and analogues, applied in the field of 2-substituted oxy-5-thiamphenicol aryl piperazine amides and their preparation, capable of solving negative symptoms and no improvement in cognitive symptoms, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: (S)-5-(4-(5-(methylsulfonyl)-2-((1,1,1-trifluoropropan-2-yl)oxo)nicotinyl)piperazine-1 Preparation of -yl)-2,3-dihydro-1H-inden-1-one
[0107] Step 1: Preparation of (S)-5-(methylsulfonyl)-2-((1,1,1-trifluoropropan-2-yl)oxo)nicotine acid
[0108]
[0109] Sodium hydride (500mg, 12.5mmoL) was dissolved in 5mL of DMF, (S)-trifluoroisopropanol (1.2g) was added, stirred at room temperature for 30 minutes, and then 2-chloro-5-bromonicotinic acid (900mg) After adding to the system, stirring at 110°C for 16 hours, spin off DMF, dissolve the residue in 10ml DMSO, add sodium proline (274mg, 2.0mmoL), sodium methanesulfinate (1.0g, 10mmoL) and sulfite Copper (761.8mg, 4.0mmoL), heated to 110°C under nitrogen atmosphere and stirred for 4 hours, separated and purified directly by reverse phase column chromatography to obtain 5-(methylsulfonyl)-2-((1,1,1-tri Fluoropropan-2-yl)oxo)nicotinic acid (150 mg).
[0110] The second step: the preparation of 5-(piperazin-1-yl...
Embodiment 2
[0119] Example 2: (S)-(4-(5-fluoro-3-methylbenzo[d]isoxazol-6-yl)piperazin-1-yl)(5-(methylsulfonyl)-2 Preparation of -((1,1,1-trifluoropropan-2-yl)oxo)phenyl)methanone
[0120] Step 1: Preparation of tert-butyl 4-(4-acetyl-2,5-difluorophenyl)piperazine-1-carboxylate
[0121]
[0122] Dissolve 2,4,5-trifluoroacetophenone (522mg, 3.0mmol) in 10mL of acetonitrile, then add N-tert-butoxycarbonylpiperazine (671mg, 3.6mmol) and potassium carbonate (829mg, 6.0mmol), Heat to reflux overnight. Separation by reverse-phase column chromatography gave compound 4-(4-acetyl-2,5-difluorophenyl)piperazine-1-carboxylic acid tert-butyl ester (670 mg, 66%). LC-MS:t R =3.12min, [M+H] + 341.2.
[0123] The second step: Preparation of tert-butyl 4-(2,5-difluoro-4-(1-(oximino)ethyl)phenyl)piperazine-1-carboxylate
[0124]
[0125] 4-(4-acetyl-2,5-difluorophenyl)piperazine-1-carboxylate tert-butyl ester (670mg, 1.97mmol), hydroxylamine hydrochloride (205mg, 2.95mmol) and sodium acetate tri...
Embodiment 3
[0137] Example 3: (S)-(4-(7-fluoroquinolin-6-yl)piperazin-1-yl)(5-(methylsulfonyl)-2-((1,1,1-trifluoro Preparation of propan-2-yl)oxo)pyridin-3-yl)methanone
[0138] The first step: the preparation of 7-fluoro-6-(piperazin-1-yl)quinoline
[0139]
[0140] Weigh 6-bromo-7-fluoroquinoline (226mg, 1.00mmol) and piperazine (129mg, 1.50mmol) and dissolve them in 5mL of 1,4-dioxane, add bis(tri-tert-butylphosphine)palladium under nitrogen protection (51 mg, 0.10 mmol) and sodium tert-butoxide (192 mg, 2.00 mmol). Microwave at 120°C for 1 hour. Add ethyl acetate to extract, wash with saturated NaCl aqueous solution, anhydrous NaCl 2 SO 4 dry. Filtration and concentration gave tan viscous liquid (210 mg, 91%), and the crude product was directly used in the next reaction.
[0141] The second step: (S)-(4-(7-fluoroquinolin-6-yl)piperazin-1-yl)(5-(methylsulfonyl)-2-((1,1,1-trifluoro Preparation of propan-2-yl)oxo)pyridin-3-yl)methanone
[0142]
[0143] Weigh the above crud...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com