Dihydro-beta-agarofuran sesquiterpenoids, and preparation method and application thereof
A technology of compounds and uses, applied in the field of dihydro-β-agarwood furan-type sesquiterpenoids, which can solve problems such as unclear natural active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Preparation and structural identification of Example 1 compound
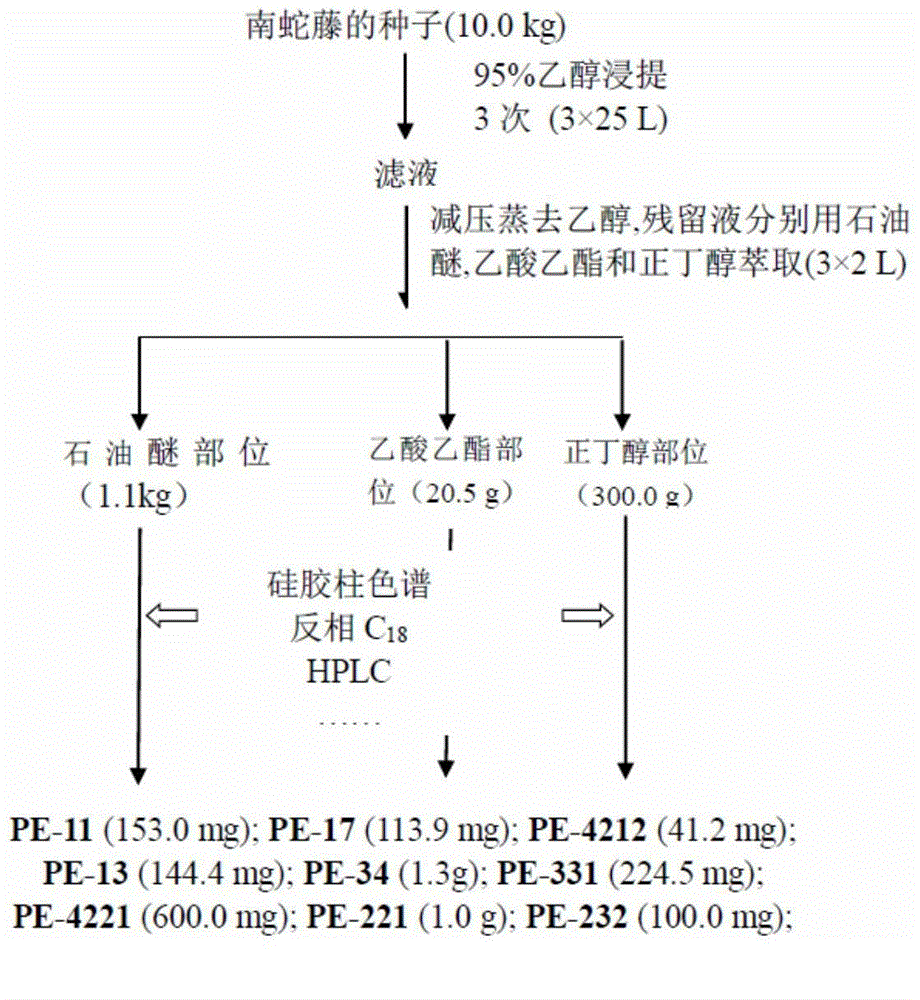
[0127] Extraction and Isolation of Compounds from the Seeds of Snakevine
[0128] The dried and pulverized C. orbiculatus seeds (C.orbiculatus) (10.0kg) were percolated three times with 95% industrial ethanol at room temperature (25L×3, 3 days / time), the filtrate was concentrated under reduced pressure to a small amount, added H 2 Suspended with petroleum ether, ethyl acetate and n-butanol (2L×3, each) in order to obtain petroleum ether fraction (1.1kg), ethyl acetate fraction (20.5g) and n-butanol fraction (100.0g) respectively . For the specific separation process of each part, see figure 1 .
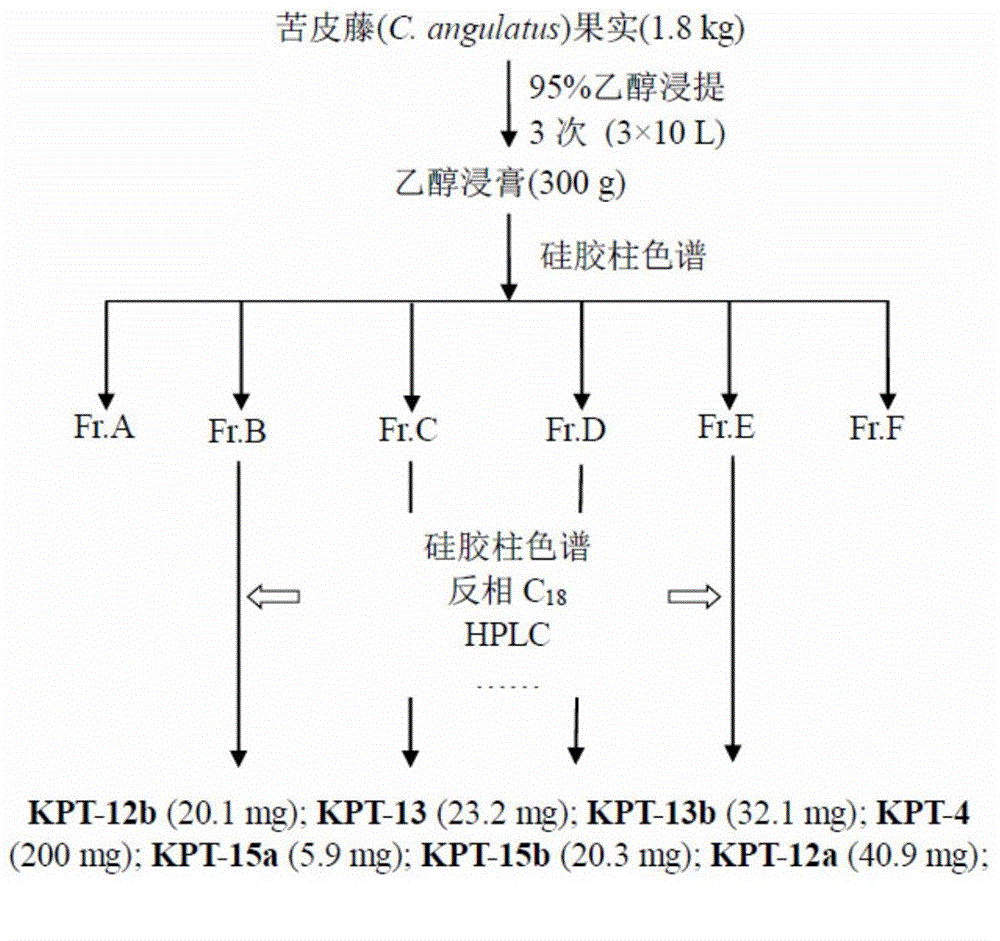
[0129] Extraction and Separation of Compounds from the Fruit of Phyllostachys chinensis
[0130] C. angulatus (C. angulatus) fruit 1.8Kg was pulverized, extracted three times (10L×3) with 95% ethanol at room temperature, evaporated under reduced pressure to remove ethanol to obtain the total extract (300g), t...
Embodiment 2
[0166] Example 2 Compounds of the present invention protect SH-SY5Y cells from Aβ 25-35 damage
[0167] This assay uses thiazolium blue (MTT) colorimetric assay to measure cell viability. Human bone marrow neuroblastoma cell line (SH-SY5Y cell) was purchased from ATCC, with MEM / F12 culture solution containing 10% fetal bovine serum, at 37oC, 5%CO 2 cultured in an incubator. When the cells grow to 80-90% density, digest with 0.125% trypsin, with 2.5*10 4 The density of cells / well was inoculated in a 96-well culture plate, and cultured in an incubator for 24 hours. Different groups were treated as follows: 24 hours after cell inoculation, the culture medium was replaced with serum-free MEM / F12 culture medium, and each compound was added (final concentration 10 μM), and the model group was added with an equal volume of solvent control. After culturing for 2 hours, Aβ25-35 with a final concentration of 10 μM was added to each treatment group, and an equal volume of culture sol...
Embodiment 3
[0173] Embodiment 3 animal memory impairment model experiment
[0174] A channel-type water maze (made of black plexiglass, 80cm×50cm×20cm, reference [JingL, ZhangHY, et al. Effects of synthetic (-)-huperzine Aoncholinesterase activities and mouse water maze performance. Acta Pharmacologica Sinica, 1998, 19, 413-416]) was used for behavioral testing. Each mouse was lightly put into the water with its nose facing the wall of the uterus, and at the same time, it began to record the time when each mouse swam to the platform and the number of times it entered the blind end before reaching the platform, as the memory performance of each mouse was evaluated. Training for 4 days, one training session in the morning and one session in the afternoon every day, with the last three training sessions within 25 seconds and the number of errors below 2 times as the standard, the mice with qualified memory scores were selected and randomly divided into groups for drug testing. The mice were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com