Detection method for ezetimibe optical isomers and application thereof
A technology for optical isomers and detection methods, applied in measurement devices, scientific instruments, instruments, etc., can solve the problem of no standard control method, and achieve the effect of high sensitivity, specificity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The choice of solvent and the choice of the concentration of the solution:
[0036] For the choice of solvent for dissolving samples, we used dimethyl sulfoxide, dimethylformamide, ethanol, isopropanol, and acetonitrile to dissolve samples respectively, all of which are easily soluble to samples. Dimethyl sulfoxide and dimethyl formamide produce large solvent peaks on the liquid chromatography column, and are likely to remain in the syringe and column to affect detection. Ethanol or isopropanol should be considered as the preferred choice. Since the n-heptane-ethanol system is selected as the mobile phase, ethanol is finally preferred as the sample solvent.
[0037] We use ethanol as the solvent of the sample, which are respectively formulated as 0.2mg / mL, 0.5mg / mL, 1.0mg / mL, 2mg / mL, 5mg / mL and 10mg / mL. Although they can be separated, the 0.2mg / mL The response is too small, and the concentration of 10mg / mL is too large to be unfavorable for the separation of the sample...
Embodiment 2
[0039] Selection of mobile phase type and flow rate:
[0040] We choose the commonly used normal phase liquid chromatography mobile phase system: n-hexane (A) - isopropanol (B), n-hexane (A) - ethanol (B), n-heptane (A) - isopropanol (B) , n-heptane (A)-ethanol (B), n-hexane (A)-tetrahydrofuran (B), n-heptane (A)-tetrahydrofuran (B); preferred composition: n-heptane (A)-ethanol (B ) screening, the results show that the main peak of the sample in the n-hexane system is wider, and the degree of separation with impurities cannot reach more than 1.5; The heptane-ethanol system is higher than the n-heptane-isopropanol system and the n-heptane-tetrahydrofuran system, so the n-heptane-ethanol system is preferred as the mobile phase system.
[0041]We have investigated the flow rate, and the technical solution can be realized in the range of 0.2-2.0mL / min, but the flow rate of 0.2mL / min is too slow, the peak time of the sample is too long, and the peak shape is too broad; the flow ra...
Embodiment 3
[0043] 1) Instruments and testing conditions
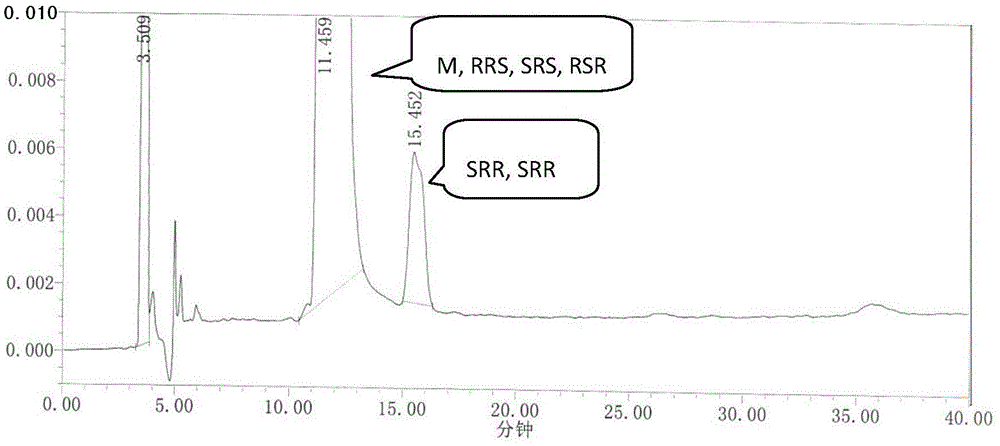
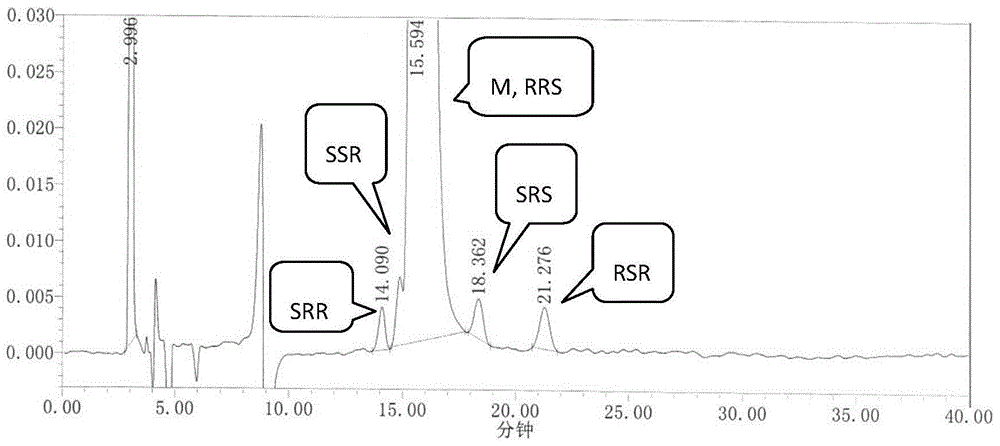
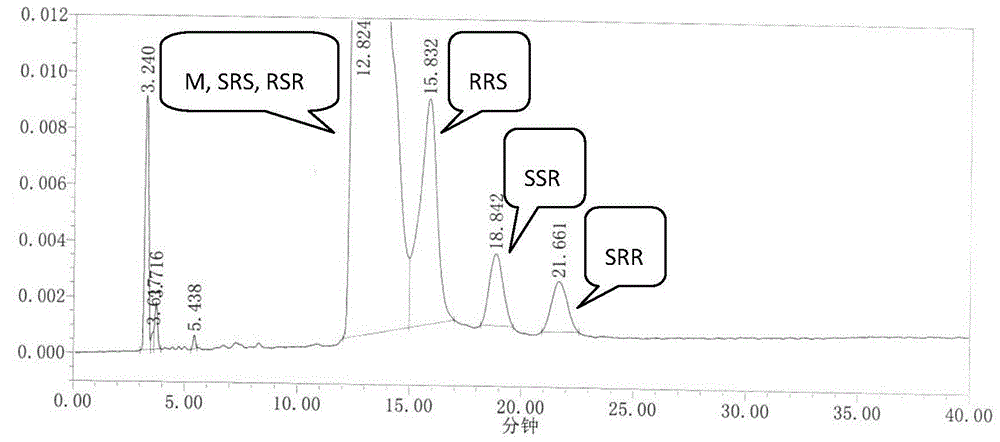
[0044] The E2695 high performance liquid chromatograph produced by U.S. Waters Company, automatic sampler, the ZorbaxNH produced by Agilent Company 2 Chiral analysis column (4.6×250mm, 5μm); mobile phase ratio n-hexane:ethanol (90:10); column temperature: 35°C; flow rate: 1.0mL / min; detection wavelength: 232nm.
[0045] 2) Experimental steps
[0046] We dissolve RRS, SSR, SRR, SRS, and RSR isomers in an appropriate amount with ethanol, add an appropriate amount of ezetimibe to the test sample, and use mobile phase to prepare 1 mg of ezetimibe per ml, containing RRS, SSR, A solution of 1 μg of SRR, SRS, and RSR isomers was used as the system suitability 1 solution. Inject 20 μL, record the chromatogram, see the results figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com