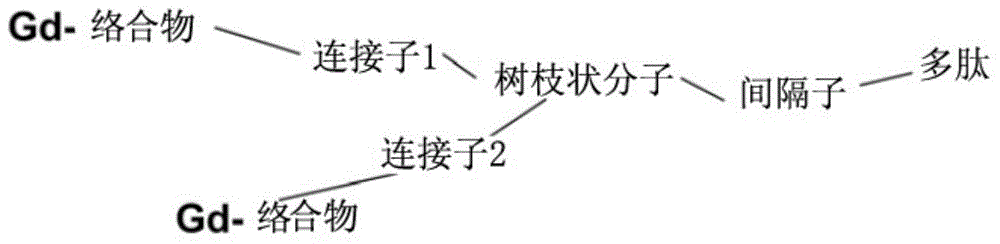
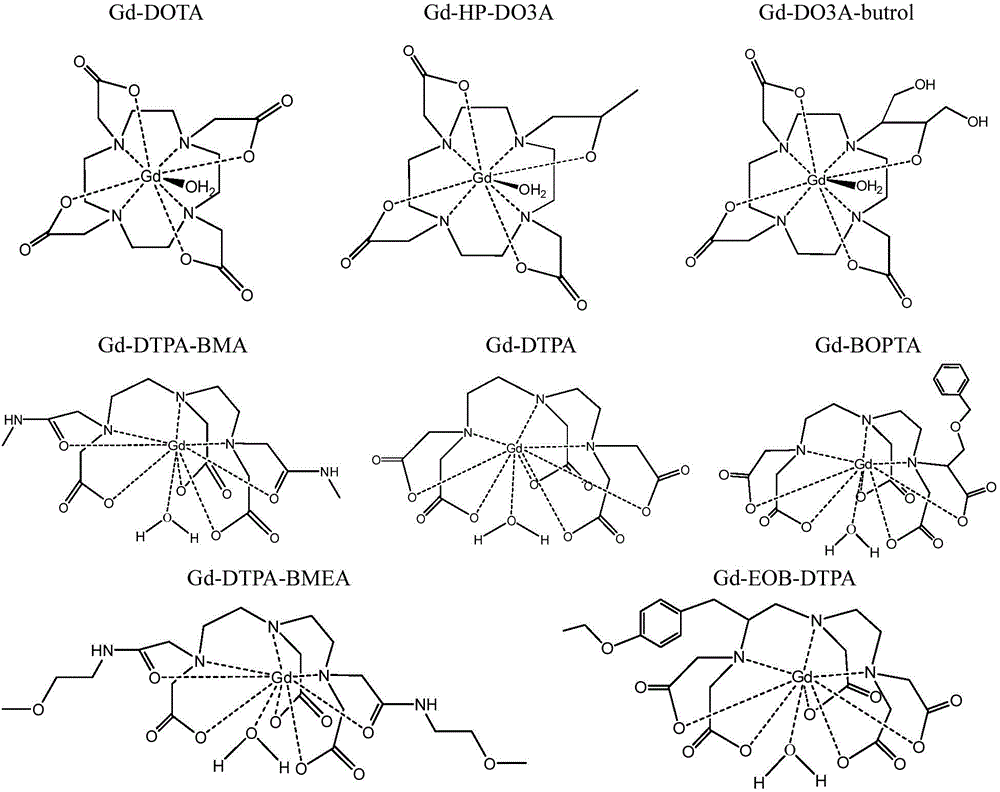
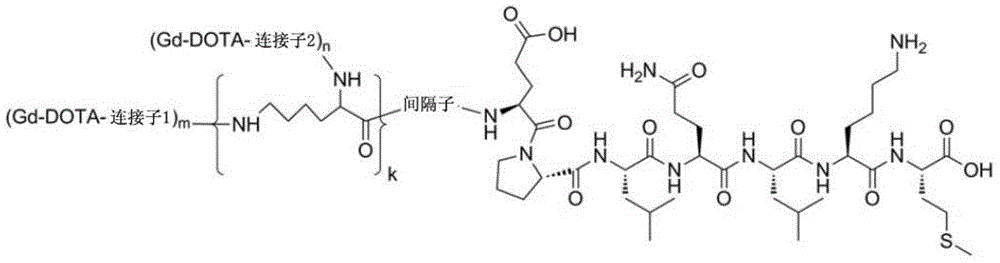
Polypeptide molecule image probe targeting mesenchymal stem cell, preparation method of polypeptide molecule image probe, and mesenchymal stem cell marked by polypeptide molecule image probe
A kind of stem cell and molecular imaging technology, applied in the field of medical imaging, can solve the problems of low relaxation rate and large dose, achieve high affinity and target specificity, reduce safety problems, and enhance the effect of magnetic resonance weighted imaging contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: (Gd-DOTA) 2-Synthesis of EM7 molecular imaging probe
[0042] 1. Bu t 3 Synthesis of DOTA (1,4,7-tris(tert-butoxycarbonylmethyl)-10-(acetic acid)-1,4,7,10-tetraazacyclododecane). The synthesis proceeds from 1,4,7,10-tetraazacyclododecane, a macrocyclic polyamine, as follows:
[0043] a) Weigh out 10.0g 1,4,7,10-tetraazacyclododecane and 29.3g NaHCO 3 In a one-liter three-necked flask, add 50 mL of acetonitrile. Weigh 37.4 g of tert-butyl bromoacetate in a fume hood, add 20 mL of acetonitrile, mix well, and put it into a dropping funnel. A solution of t-butyl bromoacetate in acetonitrile was slowly added dropwise to the reaction mixture under an ice bath and nitrogen protection. After the dropwise addition was completed, stirring was continued at room temperature for 30 hours. The solid was filtered off, the acetonitrile was removed by rotary evaporation, and recrystallized twice with toluene to obtain a white solid Bu t 3 DO3A (1,4,7-tris(tert-butoxy...
Embodiment 2
[0050] Example 2: (Gd-DOTA) 2 - Synthesis of CC9 Molecular Imaging Probe
[0051] 1. Synthetic Bu t 3 DOTA: 1,4,7-tris(tert-butoxycarbonylmethyl)-10-(acetic acid)-1,4,7,10-tetraazacyclododecane. The synthesis proceeds from 1,4,7,10-tetraazacyclododecane as follows:
[0052] a) Synthesize Bu according to Example 1 t 3 DO3A.
[0053] b) Weigh 1.38gK 2 CO 3 and 2.57gBu t 3 DO3A in a 250mL three-necked flask, add 50mL acetonitrile, N 2 Protected and stirred at room temperature, 1.37 g of benzyl bromoacetate in 5 mL of acetonitrile was added dropwise, and the reaction was carried out at 70 °C for 12-24 hours. Cool to room temperature, remove the solids by filtration, and remove the solvent by rotary evaporation. with CH 2 Cl 2 / MeOH (20:1) was used as the developing solvent for column chromatography separation, and the product 1,4,7-tris(tert-butoxycarbonylmethyl)-10-(benzyloxycarbonylmethyl) was obtained as pale yellow viscous foamy product )1,4,7,10-tetraazacyclodo...
Embodiment 3
[0059] Example 3: Preparation of Mesenchymal Stem Cells Labeled with Polypeptide Molecular Imaging Probes
[0060] 1. The polypeptide molecular imaging probe prepared in Example 1 or 2 was dissolved in the culture medium to form a concentration series of 0.05, 0.1, 0.2, 0.5, 1.0, 2.0 mM, and the mesenchymal stem cells were cultured in a D=90 mm dish 24 hours.
[0061] 2. Cell counting and cell dense packing: remove the medium, wash with PBS (2 mL×3) at pH 7.4, and remove unbound molecular imaging probes. Add 1.0 mL of trypsin for digestion, add 3 mL of PBS, and blow off the cells. Take 20 μL on a cell counting plate and count the number of cells under a microscope. All the remaining cells were transferred to a 10 mL centrifuge tube, and the culture dish was washed with 3 mL of PBS, and also transferred to the centrifuge tube. PBS was removed by centrifugation at 1200 rpm for 5 minutes. The cells were transferred to a capillary with an inner diameter of 1.5 mm and a capilla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com