HFC-125 synthesis catalyst and preparation method thereof
A HFC-125, catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Achieve the effect of good catalytic performance, high conversion rate and selectivity, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
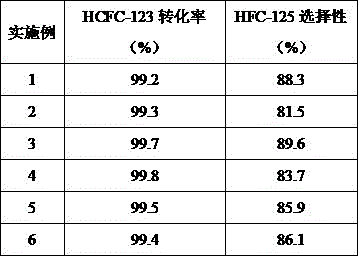
[0027] Al(NO 3 ) 3 9H 2 O8 1.80g (0.250mol), Mg(NO 3 ) 2 ·6H 2 O96.16g (0.375mol), Zn(NO 3 ) 2 ·5H 2 O111.56g (0.375mol) was prepared into 300mL mixed solution I with deionized water, and 40g of sodium hydroxide and 6.62g of sodium carbonate were prepared with deionized water into 300mL mixed solution II. Then, the mixed solution I and the mixed solution II were quickly added to the rotary liquid film reactor and mixed vigorously. After the mixing was completed, they were transferred to a 1L four-necked flask, and crystallized at 60°C for 48 hours, and washed with deionized water until neutral. After filtering, the filter cake was dried at 80°C and ground to obtain multi-component hydrotalcite. Then it was calcined at 450°C for 4h under normal pressure and under the protection of nitrogen to obtain a fluorination catalyst, which was activated by treating with anhydrous HF at 400°C for 12h before use. The reactivity and selectivity are listed in Table 1.
Embodiment 2
[0029] Al(NO 3 ) 3 9H 2 O40.90g (0.125mol), Fe(NO 3 ) 3 9H 2 O50.50g (0.125mol), Mg(NO 3 ) 2 ·6H 2 O32.05g (0.125mol), Zn(NO 3 ) 2 ·5H 2 O111.56g (0.375mol) was prepared into 300mL mixed solution I with deionized water, and 30g sodium hydroxide and 5.62g sodium carbonate were prepared with deionized water to make 300mL mixed solution II. Then, the mixed solution I and the mixed solution II were quickly added to the rotating liquid film reactor and mixed vigorously. After the mixing was completed, they were transferred to a 1L four-necked flask, and crystallized at 75°C for 36 hours, and washed with deionized water until neutral. After filtering, the filter cake is dried at 100°C and ground to obtain multi-component hydrotalcite. Then it was calcined at 300°C for 8h under normal pressure and under the protection of nitrogen to obtain a fluorination catalyst, which was activated by treating with anhydrous HF at 350°C for 24h before use. The reactivity and selectivity...
Embodiment 3
[0031] Al(NO 3 ) 3 9H 2 O58.90g (0.18mol), Fe(NO 3 ) 3 9H 2 O72.72g (0.18mol), Mg(NO 3 ) 2 ·6H 2 O92.30g (0.36mol), Zn(NO 3 ) 2 ·5H 2 O107.10g (0.36mol) was prepared into 300mL mixed solution I with deionized water, and 43.00g of sodium hydroxide and 9.39g of sodium carbonate were prepared with deionized water into 300mL mixed solution II for later use. The mixed solution I and the mixed solution II were quickly added to the rotating liquid film reactor and mixed vigorously. After the mixing was completed, they were transferred to a 1L four-necked flask, and crystallized at 100°C for 6 hours, and washed with deionized water until neutral. Filter, dry the filter cake at 105°C, and grind to obtain multi-component hydrotalcite. Then it was calcined at 550°C for 2h under normal pressure and under the protection of nitrogen to obtain a fluorinated catalyst, which was activated by treating with anhydrous HF at 300°C for 36h before use. The reactivity and selectivity are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com