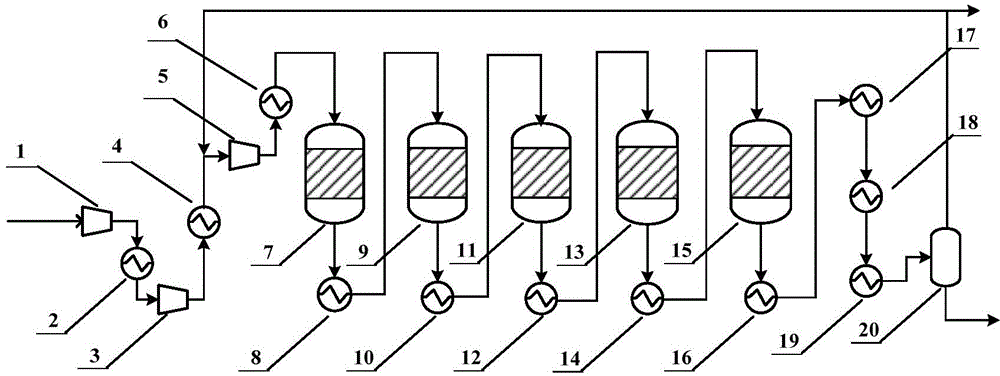
Method for preparing synthetic ammonia from low-H2/N2-ratio synthesis gas
A technology for H2-N2 and ammonia synthesis, which is applied in the preparation/separation of ammonia, production of bulk chemicals, etc., and can solve the problems of low utilization rate of synthesis gas and low ammonia yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Fresh normal temperature low pressure syngas H 2 / N 2 The ratio is 3.00. According to the calculation of the KBR three-tower process, the pressure of synthesis gas entering the first iron-based catalyst reactor is 150atm, the temperature of synthesis gas entering the first iron-based catalyst reactor is 350℃, and the synthesis gas entering the second and third reactors The temperature of each iron-based catalyst reactor is 380℃, the circulating gas purge ratio is 4%, and the catalyst loading volume of the first iron-based catalyst reactor is 15m 3 , The first iron-based catalyst reactor has a catalyst loading capacity of 25m 3 , The first iron-based catalyst reactor has a catalyst loading capacity of 40m 3 , The final export ammonia concentration is 20.92%, and the production index is shown in Table 1.
[0079] Table 1 Reaction pressure 150atm, H 2 / N 2 =3.0 iron catalyst production index value
[0080]
Embodiment 2
[0082] Fresh normal temperature low pressure syngas H 2 / N 2 The ratio is 3.00, the pressure of synthesis gas entering the iron-based catalyst reactor is 150atm, the temperature of synthesis gas entering the iron-based catalyst reactor is 350℃, the temperature of synthesis gas entering the four ruthenium-based catalyst reactors is 360℃, and the circulating gas is released The proportion is 4%, and the iron-based catalyst reactor catalyst loading capacity is 15m 3 , The first ruthenium-based catalyst reactor has a catalyst loading capacity of 5m 3 , The second ruthenium-based catalyst reactor has a catalyst loading capacity of 6m 3 , The third ruthenium-based catalyst reactor catalyst loading capacity 7m 3 , The fourth ruthenium-based catalyst reactor catalyst loading capacity 9m 3 , The final export ammonia concentration is 24.80%, and the production index is shown in Table 2.
[0083] Table 2 Reaction pressure 150atm, H 2 / N 2 =3.0 production index value of iron string ruthenium c...
Embodiment 3
[0086] Fresh normal temperature low pressure syngas H 2 / N 2 The ratio is 2.95, the pressure of synthesis gas entering the iron-based catalyst reactor is 150atm, the temperature of synthesis gas entering the iron-based catalyst reactor is 350℃, the temperature of synthesis gas entering the four ruthenium-based catalyst reactors is 360℃, and the circulating gas is released The proportion is 4%, and the iron-based catalyst reactor catalyst loading capacity is 15m 3 , The first ruthenium-based catalyst reactor has a catalyst loading capacity of 5m 3 , The second ruthenium-based catalyst reactor has a catalyst loading capacity of 6m 3 , The third ruthenium-based catalyst reactor catalyst loading capacity 7m 3 , The fourth ruthenium-based catalyst reactor catalyst loading capacity 9m 3 , Into the first ruthenium-based catalyst H 2 / N 2 =2.52, the final outlet ammonia concentration is 25.52%, and the production index is shown in Table 3.
[0087] Table 3 Reaction pressure 150atm, H 2 / N ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com