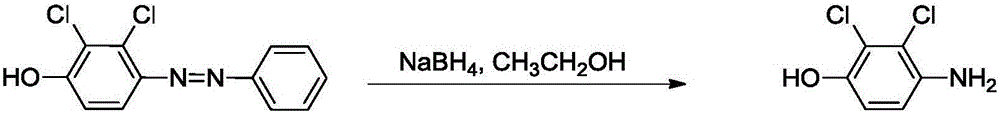
Preparation method of 2,3-dichloro-4-hydroxyaniline
A technology of hydroxyaniline and dichloro, which is applied in the field of preparation of intermediate 2,3-dichloro-4-hydroxyaniline, can solve the problems of high cost, increased risk of large-scale production, unfriendly metal waste residue, etc. Gentle, simple operation, low reaction cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of 2,3-dichloro-4-hydroxyaniline by reduction in sodium borohydride / ethanol system
[0036] In a 100 ml two-necked flask equipped with a stirring bar and a reflux condenser, add 8.0 g (0.03 moles) of raw material 2,3-dichloro-4-phenylazophenol, dissolve in 40 ml of ethanol solution, and place in an ice-water bath Add 4.5 g (0.12 mol) of sodium borohydride in small portions. After the dropwise addition was completed, the ice bath was removed under stirring, and the reaction was continued for 4 hours at room temperature. After the reaction, the dilute hydrochloric acid solution was neutralized to pH=7, extracted three times with ethyl acetate, the combined organic phase was dried with anhydrous sodium sulfate, the solvent was evaporated by a rotary evaporator, 20 ml of toluene was recrystallized, and the product was obtained by drying after filtration: 4.95 g of light pink crystals, yield 92%.
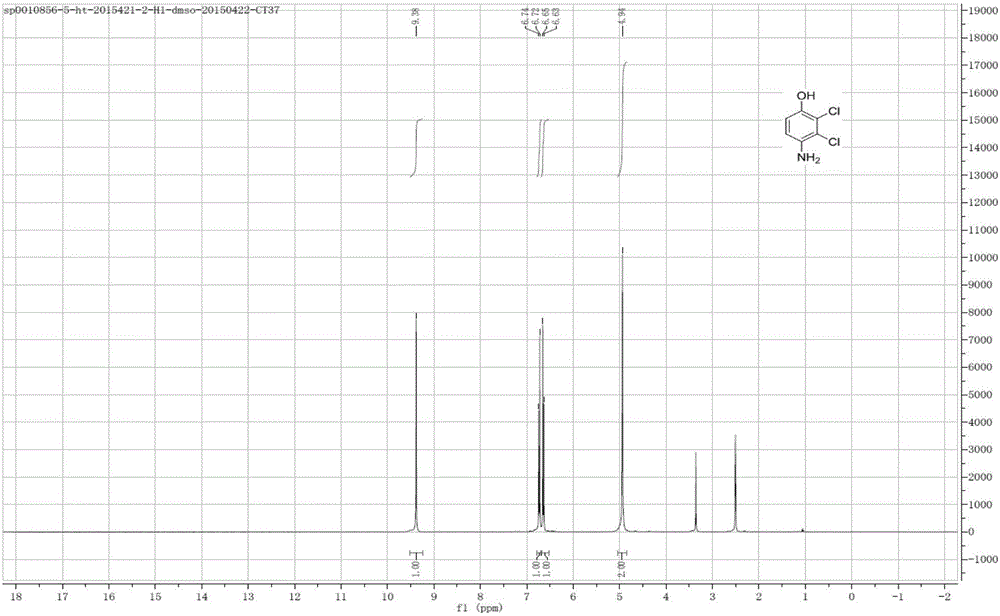
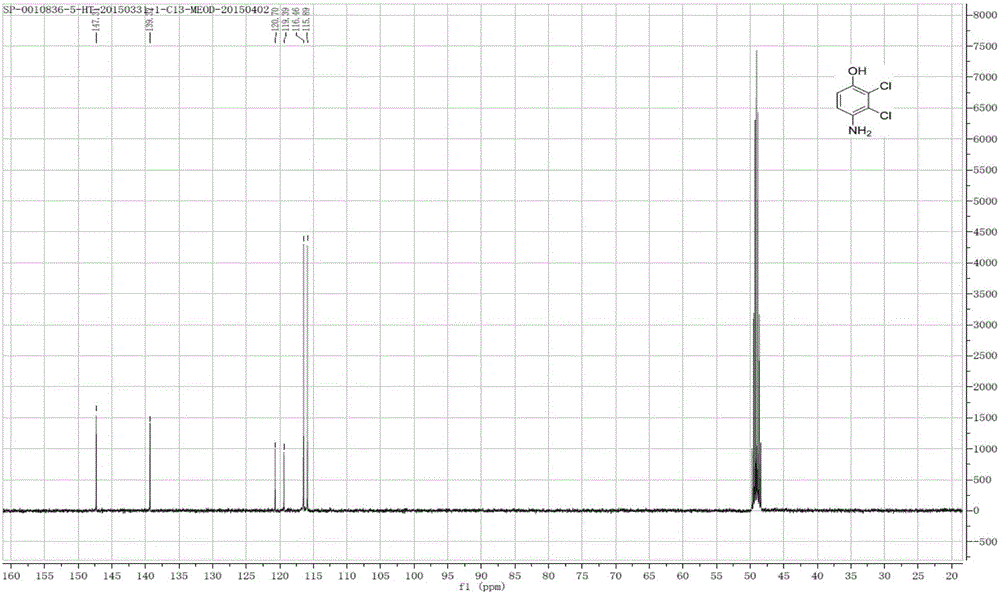
[0037] Figure 2-4 shows the 2,3-dichloro-4-hydroxyaniline 1 ...
Embodiment 2
[0039] Preparation of 2,3-dichloro-4-hydroxyaniline by reduction with sodium borohydride and sodium hydroxide / ethanol system
[0040] In a 100 ml two-necked flask equipped with a stirring bar and a reflux condenser, add 8.0 grams (0.03 moles) of raw material 2,3-dichloro-4-phenylazophenol, and dissolve 1 gram of sodium hydroxide in 40 milliliters of ethanol solution 4.5 g (0.12 mol) of sodium borohydride was added in small portions in an ice-water bath. After the dropwise addition was completed, the ice bath was removed under stirring, and the reaction was continued for 4 hours at room temperature. After the reaction, the dilute hydrochloric acid solution was neutralized to pH=7, extracted three times with ethyl acetate, the combined organic phase was dried with anhydrous sodium sulfate, the solvent was evaporated by a rotary evaporator, 20 ml of toluene was recrystallized, and the product was obtained by drying after filtration: 5.08 g of light pink crystals, yield 95%, puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com