Salt of 93beta)-17-(1H-benzimidazole-1-yl)androst-5,16-diene-3-ol and preparation method thereof
A technology of benzimidazole and androster, applied in the field of -17-androsta-5, can solve the problems of time-consuming, high dosage, complicated amorphous dispersion preparation process, etc., achieves strong economic value, high solubility, and is suitable for long-term storage and the effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] The preparation method of the phosphate crystal form A of the compound of formula (I):
[0094] Suspend 206.1 mg of the compound of formula (I) in 10 mL of acetonitrile, add 0.044 mL of 35% phosphoric acid aqueous solution, stir and react at room temperature (25±2°C) for 12 hours, and collect the solid to obtain it.
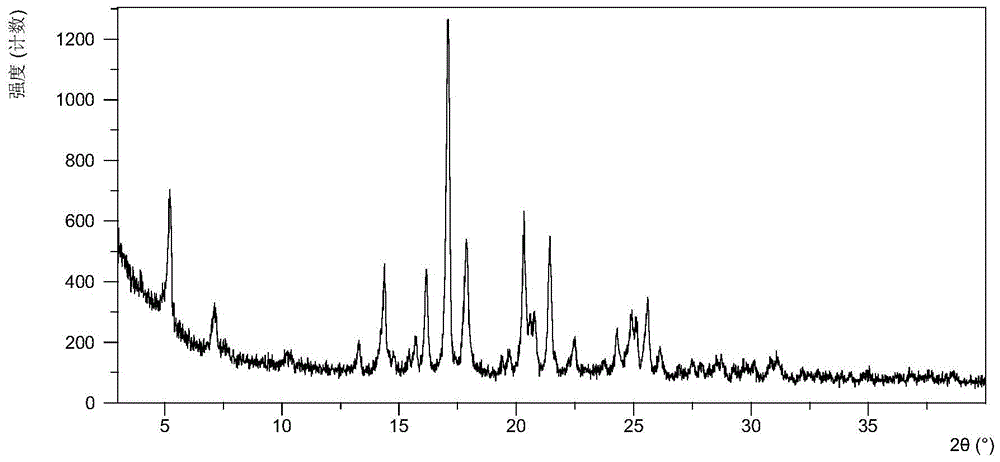
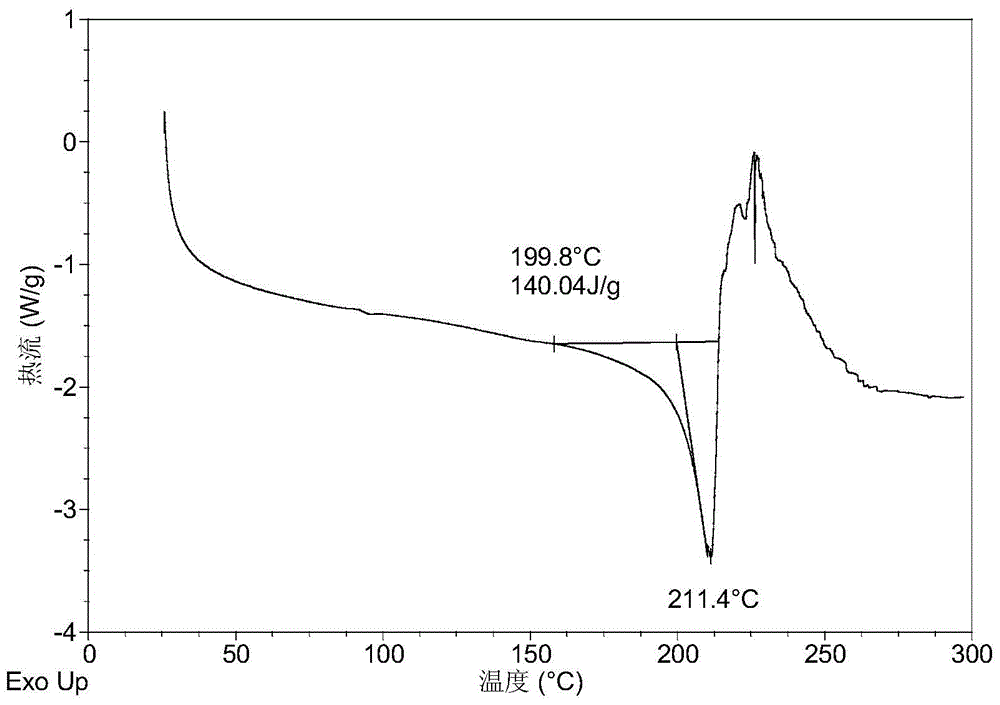
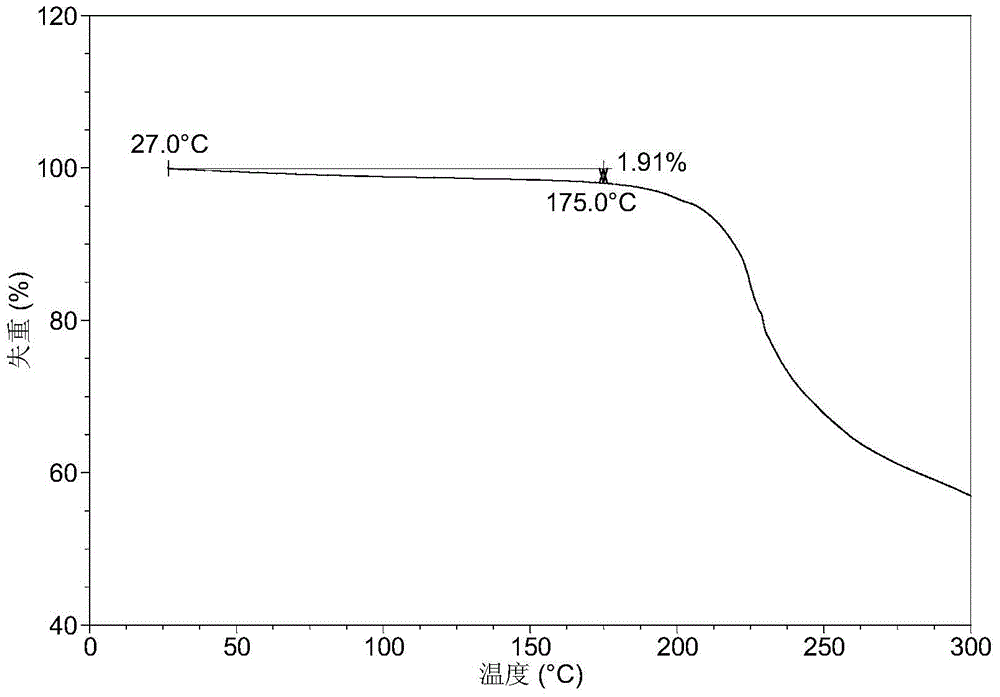
[0095] It was detected that the obtained solid was crystal form A of phosphate, and its X-ray powder diffraction data included but not limited to the data in Table 1. Its XRPD pattern is as follows figure 1 , and its DSC graph is shown in figure 2 , and its TGA figure is shown in image 3 .
[0096] The crystal form A of the phosphate salt was placed at 5°C for 90 days and then subjected to X-ray powder diffraction test. The obtained XRPD pattern is as follows Figure 4 shown.
[0097] The crystalline form A of the phosphate was placed under the conditions of 25°C and 60% relative humidity for 90 days and then subjected to X-ray powder diffraction te...
Embodiment 2
[0103] The preparation method of formula (I) compound phosphate crystal form A:
[0104] Suspend 10.1 mg of the compound of formula (I) in 0.5 mL of ethyl acetate, add 0.002 mL of 35% phosphoric acid solution, stir and react at room temperature (25±2°C) for 12 hours, and collect the solid to obtain it.
[0105] It was detected that the obtained solid was crystal form A of phosphate, and its X-ray powder diffraction data included but not limited to the data in Table 2.
[0106] Table 2
[0107]
[0108]
[0109]
Embodiment 3
[0111] The preparation method of formula (I) compound tartrate crystal form A:
[0112] Suspend 10.5 mg of the compound of formula (I) in 0.5 mL of acetone, add 5.8 mg of tartaric acid, stir and react at room temperature (25±2° C.) for 12 hours, and collect the solid.
[0113] After testing, the obtained solid is crystal form A of tartrate salt, and its X-ray powder diffraction data include but not limited to the data in Table 3. Its XRPD pattern is as follows Figure 7 , and its DSC graph is shown in Figure 8 , and its TGA figure is shown in Figure 9 .
[0114] H of tartrate crystal form A 1 -NMR picture as Figure 10 As shown, NMR data show that the salt is a compound of formula (I) and tartaric acid in a molar ratio of 1:1.
[0115] The crystal form A of the tartrate salt was placed at 5°C for 90 days and then subjected to X-ray powder diffraction test. The obtained XRPD pattern is as follows Figure 11 shown.
[0116] The crystal form A of the tartrate salt was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com