Limit test method for strychnine in Huatuo Zaizao pills
An inspection method, the technology of strychnine, applied in the field of qualitative detection of drugs, can solve the problems of ineffective control of Huatuo Zaizao pill product quality and lack of quality control methods for Nuxebra nuxe, to ensure effectiveness, safety, and operation Convenience and consistent quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
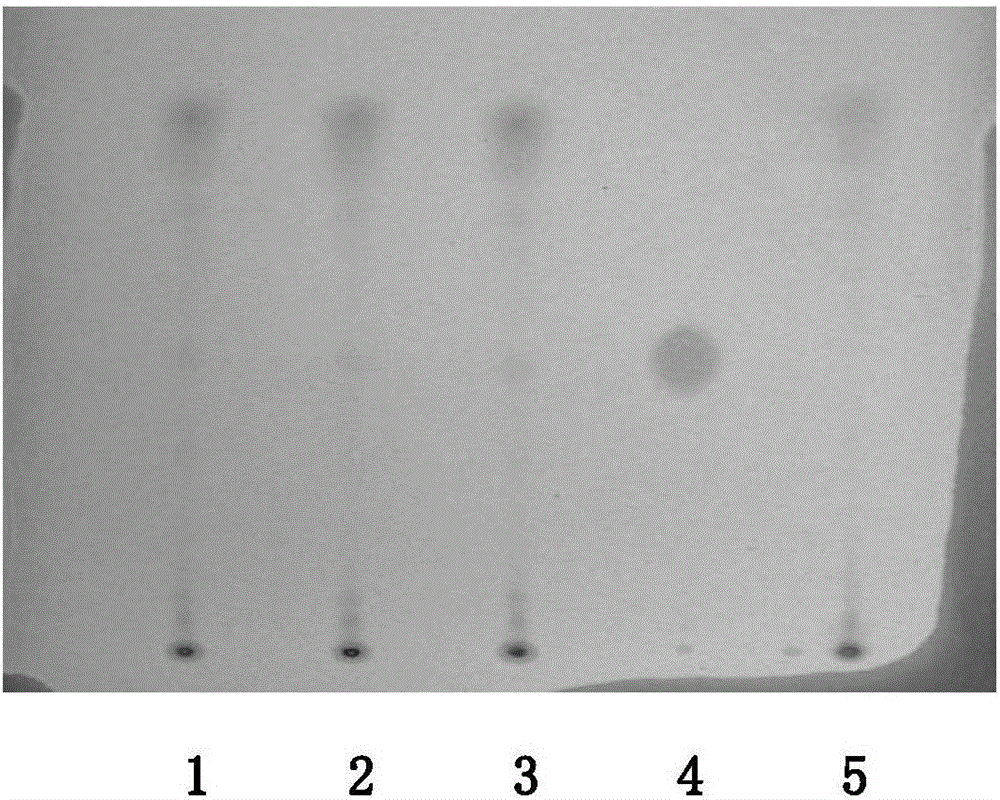
[0025] A method for checking the limit of strychnine in Huatuo Zaizao Pills uses thin-layer chromatography to measure the limit of strychnine, comprising the steps of:
[0026] Preparation of the test solution: take Huatuozaizao pills, grind them, weigh 16g of powder, then add 50ml of chloroform, 5ml of ethanol, 6ml of ammonia water, ultrasonically treat for 30-60min; filter, evaporate the filtrate to dryness, add 6% of the residue HNO 3 Heat 20ml of the solution on a water bath to dissolve, adjust the pH value to 11-12 with ammonia water, shake and extract 3 times with chloroform, 20ml each time, combine the extraction solutions, evaporate to dryness, add 1ml of chloroform to the residue to dissolve, As the test solution.
[0027] Reference substance solution preparation: take strychnine reference substance, add chloroform to make a solution containing 2mg per 1ml, as the reference substance solution.
[0028] Limit inspection: draw 12 μl of the test solution and 10 μl of t...
Embodiment 2
[0032] The characteristics of this embodiment are: the difference is that in the preparation step of the test solution: the residue is added with 6% H 2 SO 4 The solution was heated to dissolve. Other specific operation steps are the same as embodiment 1.
Embodiment 3
[0034] The characteristics of this embodiment are: the difference is that in the preparation step of the test solution: use 5% NaHCO 3 Adjust the pH value; in the limit check step: use toluene-acetone-ethanol-concentrated ammonia test solution (volume ratio 5:5:0.6:0.4) as the developing agent. Other specific operation steps are the same as embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com