Method for synthesizing 2-(4-fluorophenyl) thiophene
A technology of fluorophenyl and fluorophenylmagnesium bromide, which is applied in organic chemistry and other fields, and can solve problems such as high energy consumption, difficult purification, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Each module (comprising pre-insulation module, first reaction module, second reaction module and quenching module) in the microchannel reactor system that material flows through is replaced with nitrogen;
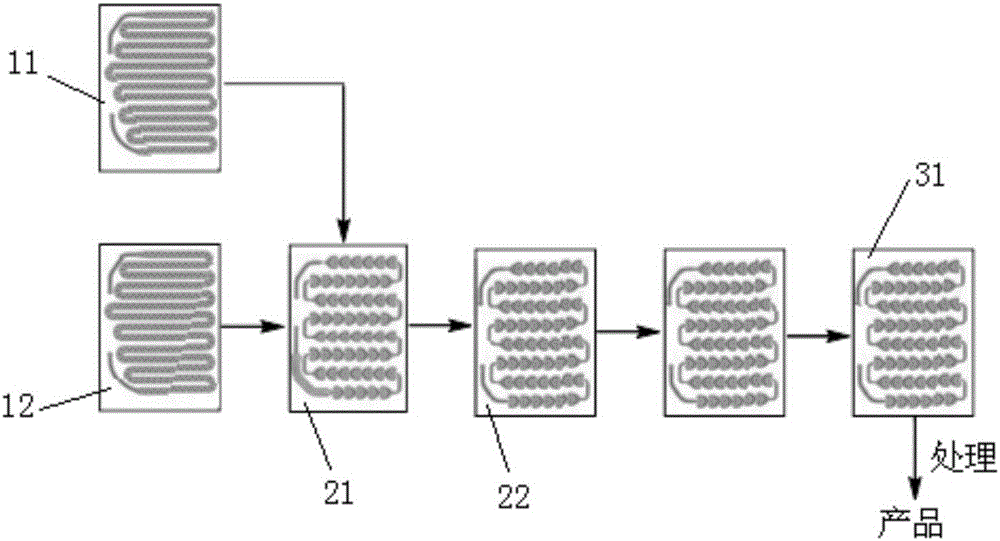
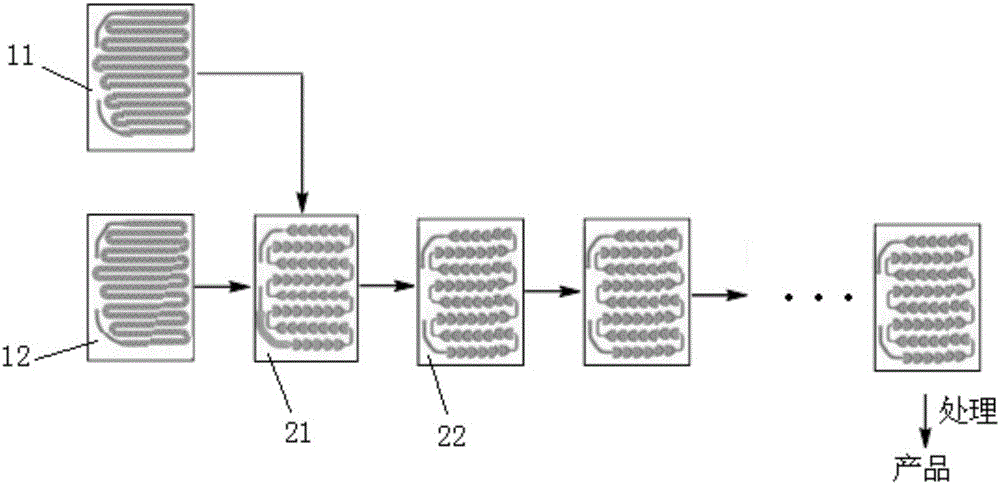
[0043] (2) Dilute 4-fluorophenylmagnesium bromide with 2-methyltetrahydrofuran to a molar concentration of 0.3mol / L as material 1; dilute trimethyl borate to 0.5mol / L with 2-methyltetrahydrofuran Homogeneous mixed solution, as material 2; Material 1 and material 2 are respectively passed into such as figure 1 The shown first pre-insulation module 11 and the second pre-insulation module 12 are pre-insulated so that both are at -5°C;
[0044] (3) After material 1 and material 2 are pre-insulated, they are all passed into the first reaction module, such as figure 1 As shown, material 1 and material 2 are mixed and reacted in the first reaction module 21, then continue to react in two second reaction modules 22, the reaction temperature in the first reaction module ...
Embodiment 2
[0050] (1) Each module (comprising pre-insulation module, first reaction module, second reaction module and quenching module) in the microchannel reactor system that material flows through is replaced with nitrogen;
[0051] (2) Dilute 4-fluorophenylmagnesium bromide with tetrahydrofuran to a molar concentration of 0.4mol / L as material 1; dilute trimethyl borate with tetrahydrofuran to a homogeneous mixed solution of 0.5mol / L as material 2; Pass material 1 and material 2 respectively into such as figure 1 The shown first pre-insulation module 11 and the second pre-insulation module 12 are pre-insulated so that they are both at 0°C;
[0052] (3) After material 1 and material 2 are pre-insulated, they are all passed into the first reaction module, such as figure 1 As shown, material 1 and material 2 are mixed and reacted in the first reaction module 21, and then continue to react in two second reaction modules 22, and the reaction temperature in the first reaction module and th...
Embodiment 3
[0058] (1) Each module (comprising pre-insulation module, first reaction module, second reaction module and quenching module) in the microchannel reactor system that material flows through is replaced with nitrogen;
[0059] (2) Dilute 4-fluorophenylmagnesium bromide with tetrahydrofuran to a concentration of 0.3mol / L as material 1; dilute trimethyl borate with tetrahydrofuran into a homogeneous mixed solution of 0.4mol / L as material 2; Pass material 1 and material 2 respectively into such as figure 1 The shown first pre-insulation module 11 and the second pre-insulation module 12 are pre-insulated so that both are at -5°C;
[0060] (3) After material 1 and material 2 are pre-insulated, they are all passed into the first reaction module, such as figure 1As shown, material 1 and material 2 are mixed and reacted in the first reaction module 21, then continue to react in two second reaction modules 22, the reaction temperature in the first reaction module and the two second reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com