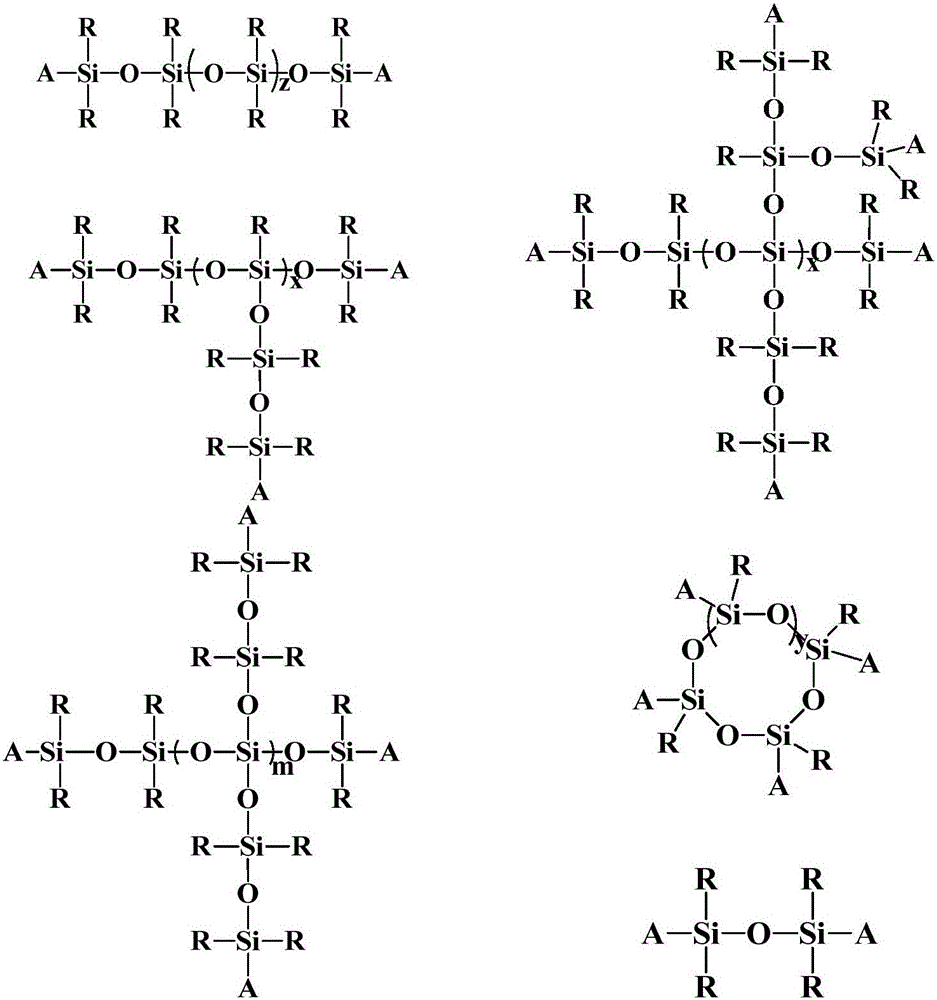Reactive hyperbranched siloxane resin as well as preparation method and application thereof
A technology of hyperbranched siloxane and base siloxane, applied in the direction of coating, etc., can solve the problems of unfavorable addition, high viscosity of resin, becoming solid, etc., and achieve the effect of promoting chain transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
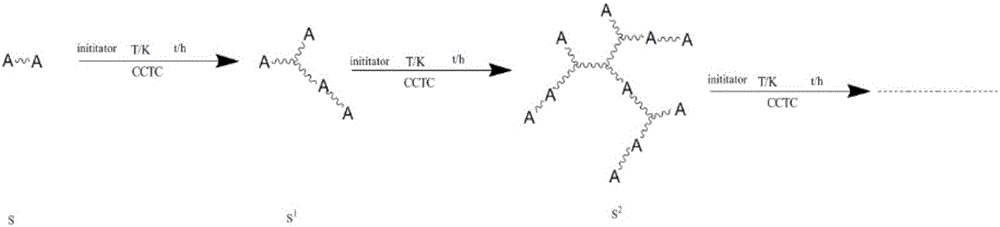
[0052] Embodiment 1: the preparation of active hyperbranched silicone resin
[0053] Weigh 1000 parts of divinyltetramethylsiloxane (M vi m vi ) (Shanghai Aladdin Biochemical Technology Co., Ltd.), 3×10 -3 Parts of cobalt II oxime boron fluoride complex (CoBF) (Nanjing Genesis New Material Co., Ltd.), 1g of azobisisobutyronitrile (AIBN) (Shanghai Aladdin Biochemical Technology Co., Ltd.) were placed in a 2L container In a single-necked round bottom flask, vacuumize, fill with high-purity nitrogen to remove oxygen, repeat the cycle 5 times, seal it, and place it in an oil bath at 50°C for 2.5 hours. After the reaction, cool the reaction solution, and then vacuumize the system , Dispose of unreacted divinyltetramethylsiloxane (M vi m vi ), then wash with anhydrous methanol, follow the principle of a small amount of multiple times, and finally through centrifugation, the methanol supernatant is dumped, and the product obtained is then vacuumized to obtain an active hyperbranc...
Embodiment 2
[0054] Embodiment 2: the preparation of active hyperbranched silicone resin
[0055] Weigh 1000 parts of divinyltetramethylsiloxane (M vi m vi ) (Shanghai Aladdin Biochemical Technology Co., Ltd.), 3×10 -3 Parts of cobalt II oxime boron fluoride complex (CoBF) (Nanjing Genesis New Material Co., Ltd.), 1g of azobisisobutyronitrile (AIBN) (Shanghai Aladdin Biochemical Technology Co., Ltd.) were placed in a 2L container In a single-necked round-bottomed flask, vacuumize, fill with high-purity nitrogen and deoxygenate repeatedly for 5 times, seal it, and place it in a 75°C oil bath to react for 2.5 hours. After the reaction is completed, cool the reaction solution, and then vacuumize the system. Dispose of unreacted divinyltetramethylsiloxane (M vi m vi ), then wash with anhydrous methanol, follow the principle of a small amount of multiple times, and finally through centrifugation, the methanol supernatant is dumped, and the product obtained is vacuumized to obtain active hyp...
Embodiment 3
[0056] Embodiment 3: the preparation of active hyperbranched silicone resin
[0057] Weigh 1000 parts of divinyltetramethylsiloxane (M vi m vi ) (Shanghai Aladdin Biochemical Technology Co., Ltd.), 9×10 -3 1 part of CoBF and 1g of azobisisobutyronitrile (AIBN) (Shanghai Aladdin Biochemical Technology Co., Ltd.) were placed in a 2L single-necked round bottom flask, vacuumed, filled with high-purity nitrogen and deoxygenated repeatedly for 5 times, and sealed. , placed in an oil bath at 100°C for 2.5 hours, after the reaction, the reaction liquid was cooled, and then the system was vacuumized to remove unreacted divinyltetramethylsiloxane (M vi m vi ), then wash with anhydrous methanol, follow the principle of a small amount of multiple times, and finally through centrifugation, the methanol supernatant is dumped, and the product obtained is vacuumized to obtain active hyperbranched silicone resin at last, adding a small amount of Hydroquinone is easy to store.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com