A kind of exenatide sustained-release microsphere composition and preparation method thereof
A technology of exenatide and sustained-release microspheres, which is applied in the field of exenatide sustained-release microsphere compositions and the preparation thereof, can solve the problems of difficulty in retaining drug activity, complicated preparation process, low encapsulation rate, etc. The effect of avoiding the burst effect, simplifying the preparation process, and avoiding the loss of activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
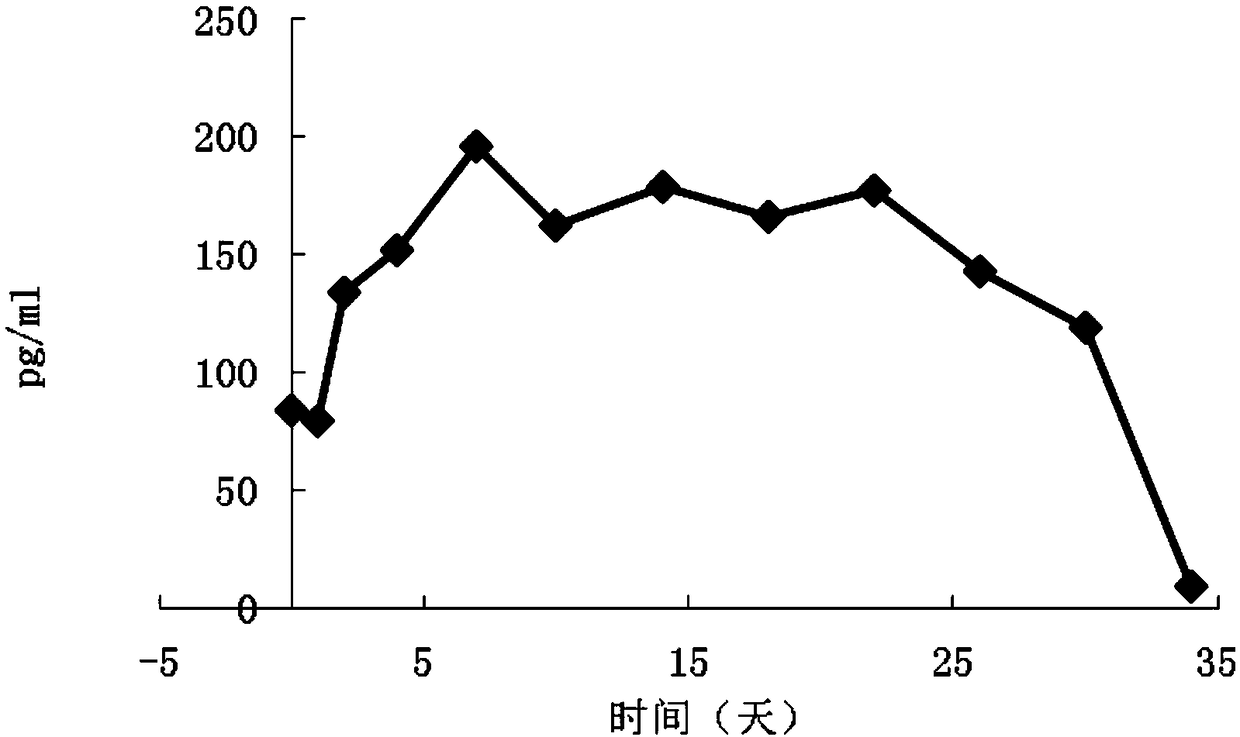
Examples
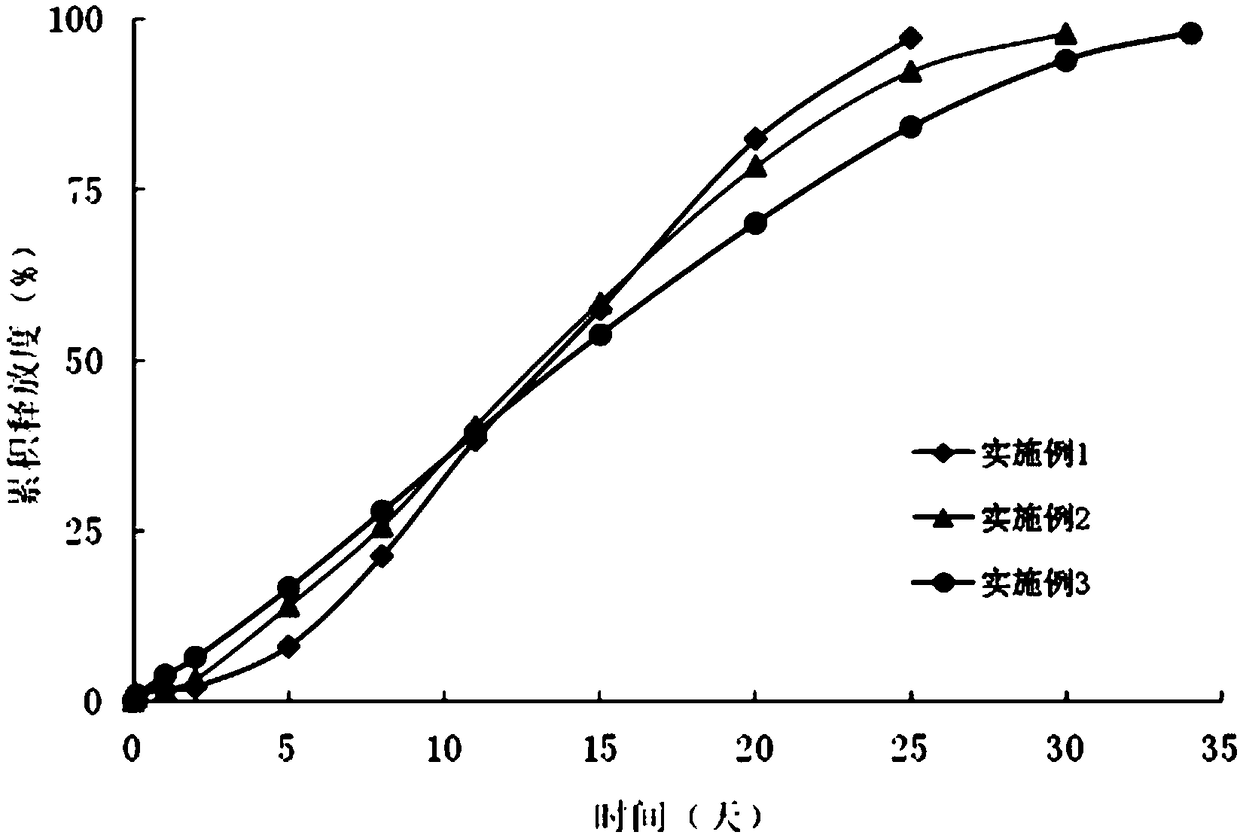
experiment example 1
[0031] Experimental Example 1 Condition screening test for preparation of exenatide microsphere composition of the present invention (screening of particle size in suspension) 50 mg of exenatide was dissolved in 2 ml of glacial acetic acid, and 0.62 g of purified 50:50DLG3A PLGA was dissolved in 9ml of dichloromethane to form a polymer solution. After mixing the glacial acetic acid solution of exenatide with the dichloromethane solution dissolved in PLGA (polylactic acid-glycolic acid copolymer), the prepared S / O The particle diameters of the drug particles in the type drug suspension are respectively 4 μm, 2 μm, 1 μm, 0.3 μm, and 0.1 μm (the particle diameter of the suspension is measured by a Malvern ZEN1690-nanometer particle size analyzer), and other operations are basically the same as in Example 1. For the senatide sustained-release microspheres, the initial release degrees of the microspheres prepared by using the in vitro release assay method (4) in Experimental Example...
experiment example 2
[0032] Experimental example 2 Condition screening test (strongly polar solvent screening test) for the preparation of exenatide microsphere composition of the present invention
[0033] Dissolve 20mg of exenatide in 2ml of dimethyl sulfoxide or 2ml of glacial acetic acid or 2ml of N,N-dimethylformamide or 2ml of methanol, and mix it with the dichloromethane solution of PLGA, the particles in the suspension The particle size is 0.3 μm, and other operations are basically the same as in Experimental Example 1. Exenatide slow-release microspheres are prepared, and the encapsulation efficiency of the prepared microspheres is detected by the method for measuring the encapsulation efficiency in (2) in Experimental Example 5. 91.2%, 89.6%, 36.4%, and 42.7%. In order to meet higher encapsulation efficiency, the preferred strong polar solvent is dimethyl sulfoxide and glacial acetic acid, more preferably dimethyl sulfoxide.
experiment example 3
[0034] Experimental Example 3 Condition screening test for preparation of exenatide microsphere composition of the present invention (screening of strong polar solvent / weak polar solvent ratio)
[0035] Taking dimethyl sulfoxide as an example to carry out the screening experiment, dissolving exenatide in different volumes of dimethyl sulfoxide solution, the ratio of dimethyl sulfoxide to methylene chloride solution is 1:5, 1:10 respectively , 1:20, 1:30, 1:40, 1:50, other operations are basically the same as in Experimental Example 2, prepare exenatide sustained-release microspheres, and adopt the in vitro release assay method of (4) in Experimental Example 5 to measure The encapsulation efficiencies of the prepared microspheres were 30.2%, 44.9%, 86.1%, 92.2%, 89.4%, 49.4%, respectively. In order to meet a higher encapsulation efficiency, the preferred volume ratio of dimethyl sulfoxide to dichloromethane solution is 1:20 to 1:40, more preferably 1:30.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com