Calixarene amide derivative stationary phase and preparation method and application thereof
An aromatic hydrocarbon amide and derivative technology, which is applied in the field of a calixarene amide derivative stationary phase and its preparation, can solve the problems of low separation efficiency and low separation purity, and achieves a simple preparation method, good application prospect and high bonding amount. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
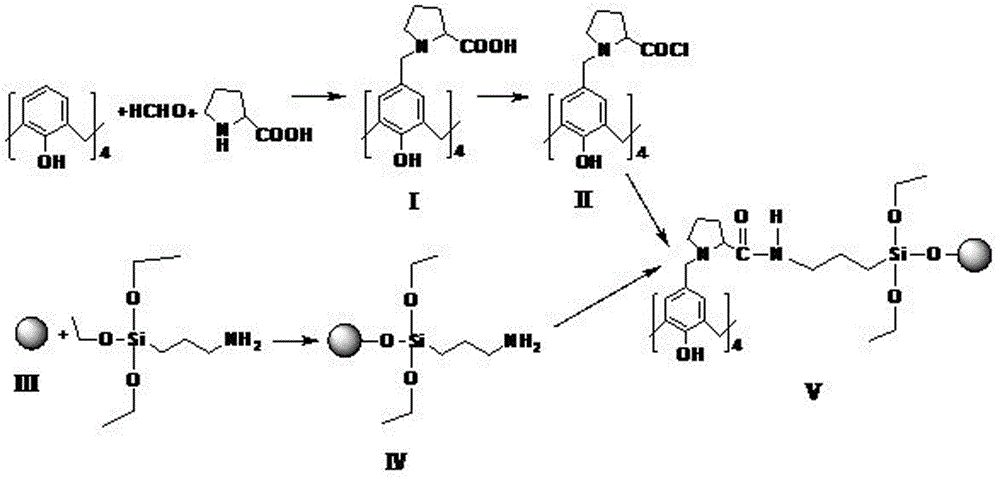
[0024] like figure 1 As shown, the stationary phase of a calixarene amide derivative provided in this example includes a calixarene group, a proline group and an aminopropyltriethoxy bonded silica gel group, and its structural formula is shown in the following formula, The spheres represent the activated silica chains:
[0025] .
[0026] The calixarene amide derivative stationary phase described in this embodiment can be passed such as figure 2 Shown reaction process obtains, and concrete preparation process comprises the following steps:
[0027] Synthesis of calixarene proline derivatives (I): Take 0.8g of de-tert-butylcalixarene in a 50mL round bottom flask, add 20mL of tetrahydrofuran solution under magnetic stirring, then add 1g of L-proline, 2mL of Glacial acetic acid and 1 mL of formaldehyde aqueous solution with a mass fraction of 37% were reacted at room temperature for 36 hours, filtered and washed 5 times with tetrahydrofuran, and the obtained solid was recry...
Embodiment 2
[0037] The calixarene amide derivative stationary phase provided in this example has the same structural formula as that in Example 1.
[0038] This embodiment also provides a preparation method for preparing the stationary phase of the calixarene amide derivative, the whole reaction process is as follows figure 2 As shown, the specific preparation process is roughly the same as the preparation steps in Example 1, except that:
[0039] In the synthesis step of calixarene proline derivatives (I), 1.5 g of de-tert-butyl calixarene dissolved in 40 mL of tetrahydrofuran solution and 5 g of L-proline, 3 mL of glacial acetic acid and 2 mL of mass percent are 30% aqueous formaldehyde solution was used for the reaction.
[0040] In the acid chlorination step of the calixarene proline derivative, 1.9 g of the calixarene proline derivative was reacted with 3 mL of oxalyl chloride in anhydrous dichloromethane.
[0041] In the preparation step of aminopropyltriethoxysilane-bonded silic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com