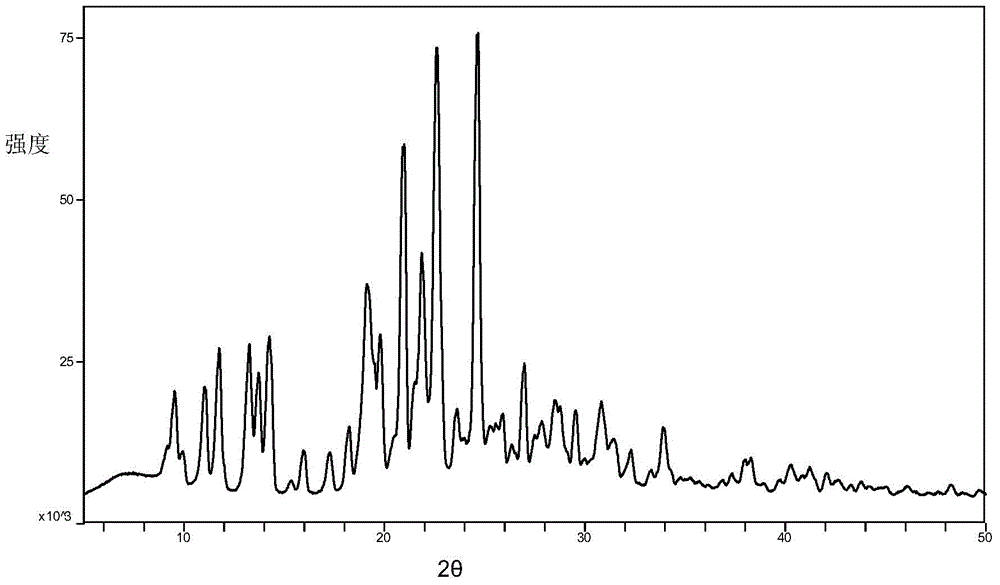
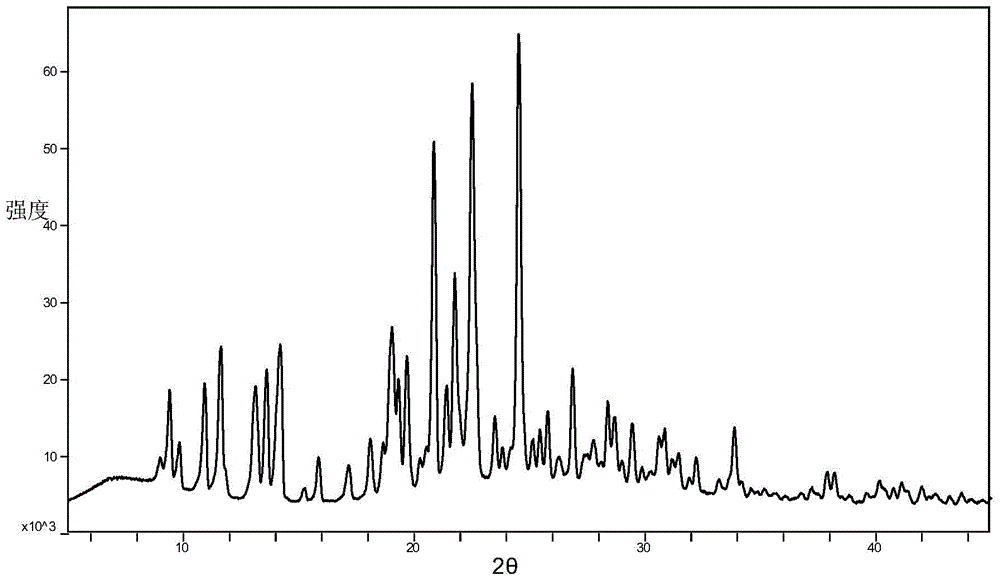
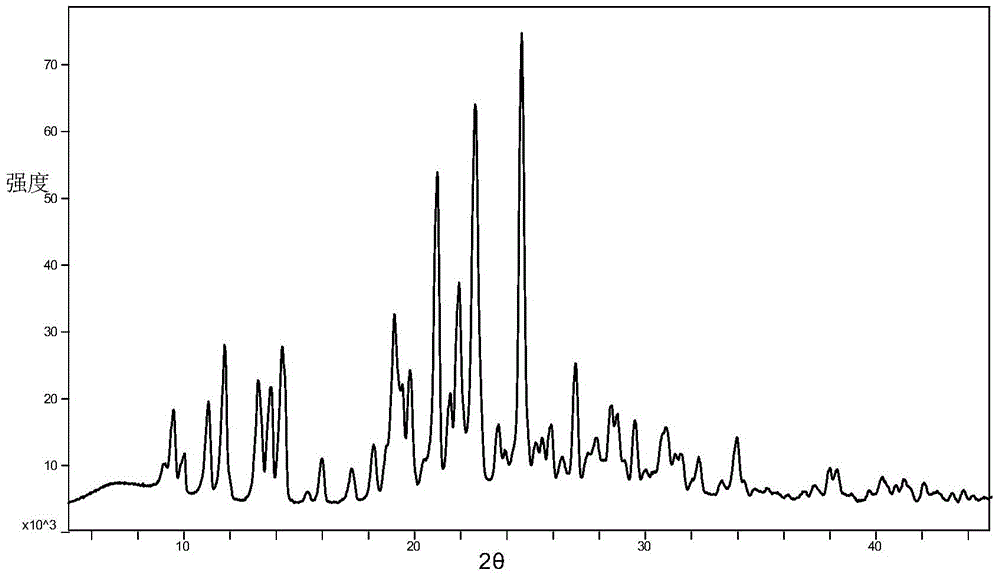
Azilsartan alkali salt A crystal form, preparation method and application thereof
A technology of choline salt and crystal form, applied in the field of Azisartan choline salt crystal form A and its preparation, and achieves remarkable effects of stability and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 500 mg of azilsartan choline salt raw material powder in a container, add 100 ml of organic solvent, suspend at a temperature of 37°C for 48 hours, then filter and vacuum dry to obtain a white powder that is azilsartan choline salt A Crystal form, wherein, the ratio of azilsartan and choline in the raw material of azilsartan choline salt is 2:1.
[0034] The purity grades of the organic solvents used in the above method are all analytically pure grades, which can be ethanol, n-propanol, n-butanol, isopropanol, isopropyl ether, methyl tert-butyl ether, acetone, ethyl acetate, Any one of isopropyl acetate, 4-methyl-2-pentanone, 2-butanone, sec-butanol, n-heptane, acetonitrile, toluene, dichloromethane and tetrahydrofuran, or any two or two of the above Mix more than one in any proportion.
Embodiment 2
[0036] Dissolve 500 mg of azilsartan choline salt raw material powder in 10 ml of methanol, add n-propanol, isopropanol, diethyl ether, isopropyl ether, 4-methyl-2-pentanone, methyl tert-butyl ether Or any single solvent in acetonitrile until a small amount of solid precipitation stops, leave it for 12 hours to obtain a white solid, filter and vacuum-dry the white powder obtained by the white solid, which is the Azilsartan choline salt A crystal form, wherein , the ratio of azilsartan and choline in the raw material of azilsartan choline salt is 2:1.
[0037] In this example, the purity grades of methanol, n-propanol, isopropanol, diethyl ether, isopropyl ether, 4-methyl-2-pentanone, methyl tert-butyl ether or acetonitrile are analytical grades.
[0038] The ratio selection of azilsartan and choline in the azilsartan choline salt raw material is the same as in Example 1.
Embodiment 3
[0040] 500 mg of azilsartan choline salt raw material powder is dissolved in 20 milliliters of dimethylformamide, any single solvent is added in isopropyl ether, methyl tert-butyl ether or acetonitrile until a small amount of solids are separated out and stopped, static Set aside for 12 hours to obtain a white solid, filter and vacuum-dry the white solid to obtain a white powder that is the Azilsartan choline salt A crystal form, wherein the azilsartan choline salt raw material is azilsartan and choline The ratio is 2:1.
[0041] In this example, the purity grades of dimethylformamide, isopropyl ether, methyl tert-butyl ether or acetonitrile are all analytical grades.
[0042] The ratio selection of azilsartan and choline in the azilsartan choline salt raw material is the same as in Example 1.
[0043] The above crystal form A of azilsartan choline salt provided by the present invention can be used to prepare medicines for treating hypertension.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com