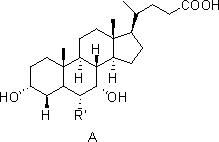
A kind of method for preparing chenodeoxycholic acid analog
A technology for cholanoic acid and cholanoate, which is applied in the field of organic synthesis of medicines and achieves the effects of simple process operation, high yield and avoiding ultra-low temperature reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
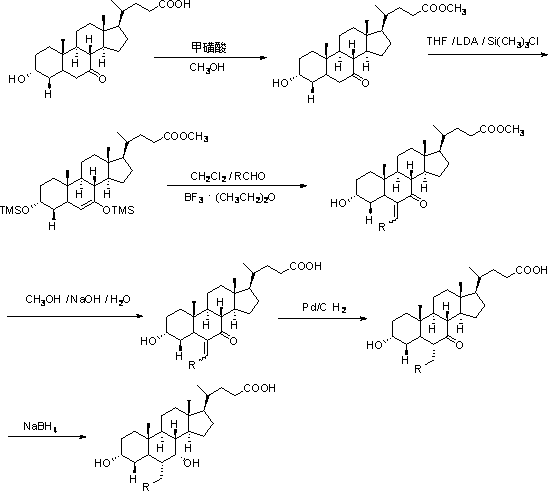
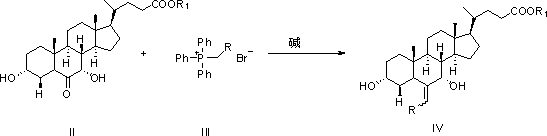
Image
Examples
Embodiment 1
[0046] Suspend 4.8g of potassium tert-butoxide (0.043mol) in 50ml of tetrahydrofuran at room temperature, add 16.0g of ethyl triphenylphosphine bromide (0.043mol) under stirring, the reaction solution turns blood red, stir for 30 minutes and then add dropwise A solution of 16.3 g of 3α,7α-dihydroxy-6-keto-5β-cholanoic acid methyl ester (0.040 mol) in 50 ml of tetrahydrofuran was reacted at room temperature for 2 hours, and the reaction was monitored by TLC. After the reaction is completed, add 100ml of water and adjust the pH value to 6~7 with 2M hydrochloric acid, add 200ml of ethyl acetate to extract, separate the liquids, and evaporate the solvent under reduced pressure to obtain 18.9g of the oily product 3α,7α-dihydroxy-6-vinyl- 5β-methyl cholanoate, this product is directly subjected to the next reaction.
[0047]
[0048] 3α,7α-Dihydroxy-6-vinyl-5β-cholanoic acid methyl ester was analyzed by mass spectrometry, H NMR spectrum and carbon spectrum, and the following result...
Embodiment 2
[0053] Dissolve 18.9 g of the oily product obtained in Example 1 in 80 ml of methanol, add 8.0 g of 40% sodium hydroxide solution, heat to 30-40 ° C for about 1 hour, and monitor the reaction by TLC. After the reaction is completed, add 150ml of water and adjust the pH value to 4~5 with 2M hydrochloric acid, a large amount of white precipitates are formed, crystallize at about 10°C for 1 hour, filter, wash the filter cake with 50ml of water, and dry to obtain 3α,7α-dihydroxy - 14.8g of crude product of 6-vinyl-5β-cholanic acid. The resulting crude product was recrystallized with 50 ml of butyl acetate and dried to obtain 13.2 g of white powdery solid 3α, 7α-dihydroxy-6-vinyl-5β-cholanic acid, with an HPLC purity of 96.6%. The starting material 3α,7α- The calculated yield of methyl dihydroxy-6-keto-5β-cholanate was 81.0%.
[0054]
[0055] 3α,7α-dihydroxy-6-vinyl-5β-cholanic acid was analyzed by mass spectrometry, H NMR spectrum and carbon spectrum, and the following result...
Embodiment 3
[0060] Suspend 13.2g of 3α, 7α-dihydroxy-6-vinyl-5β-cholanic acid in 80ml of water, add 15g of 20% sodium hydroxide solution, stir to dissolve, then add 1g of 10% palladium carbon, heat to 80 °C, hydrogen gas was introduced to react at 3 atmospheres, and the reaction progress was monitored by HPLC. After the reaction is completed, cool down to room temperature, remove the catalyst by filtration, adjust the pH value to 4~5 with 2M hydrochloric acid, a large amount of white precipitates are formed, crystallize at 0°C~5°C for 1 hour, filter, wash the filter cake with 50ml of water, and dry 12.8 g of crude 3α,7α-dihydroxy-6α-ethyl-5β-cholanic acid was obtained. The obtained crude product was recrystallized with 50 ml of butyl acetate and dried to obtain 11.5 g of white powdery solid 3α,7α-dihydroxy-6α-ethyl-5β-cholanic acid, with an HPLC purity of 98.3% and a yield of 87.1%.
[0061]
[0062] 3α,7α-Dihydroxy-6α-ethyl-5β-cholanic acid was analyzed by mass spectrometry, H-NMR an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com