A kit for evaluating sperm quality after in vitro capacitation and using method thereof
A kit and sperm technology, applied in the field of medical testing, can solve the problems of complex technical process, unscientific enzyme activity value, different enzyme activity value, etc., and achieve the effect of simple operation method and objective test results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
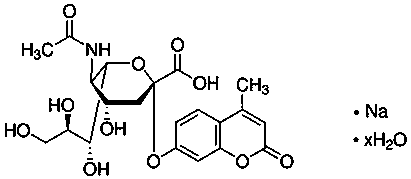
[0042] A kit for assessing sperm quality after in vitro capacitation, said kit including detection buffer 1, BWW culture fluid, in vitro capacitation solution, fluorescent reagent, sialidase standard stock solution and detection buffer 2; The detection buffer 1 is a phosphate buffer; the detection buffer 2 is a phosphate buffer containing 0.05 mmol / l sodium acetate and 0.25 μg / μl bovine serum albumin.
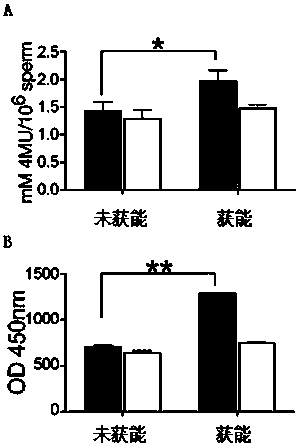
[0043] The kit of the present invention respectively measures the sialidase activity of non-capacitated sperm and capacitated sperm (A is mouse, B is human), and the results show that the capacitated sperm can release sialidase into the capacitation fluid.
Embodiment 2
[0045] A kit for assessing sperm quality after in vitro capacitation, said kit including detection buffer 1, BWW culture fluid, in vitro capacitation solution, fluorescent reagent, sialidase standard stock solution and detection buffer 2; The detection buffer 1 is BWW culture medium; the detection buffer 2 is a phosphate buffer containing 0.05 mmol / l sodium acetate and 0.25 μg / μl bovine serum albumin.
[0046] The in vitro capacitation solution is HTF containing 5 mg / ml HSA.
Embodiment 3
[0048] A kit for assessing sperm quality after in vitro capacitation, said kit including detection buffer 1, BWW culture fluid, in vitro capacitation solution, fluorescent reagent, sialidase standard stock solution and detection buffer 2; The detection buffer 1 is a phosphate buffer; the detection buffer 2 is a phosphate buffer containing 0.05 mmol / l sodium acetate and 0.25 μg / μl bovine serum albumin.
[0049] The in vitro capacitation solution is HTF containing 5 mg / ml HSA.
[0050] The sialidase standard stock solution is Clostridium perfringens sialidase with a concentration of 5 U / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com