Grass carp bacterial septicemia and grass carp bacterial red skin disease bigeminy propolis inactivated vaccine and preparing technology
An inactivated vaccine, bacterial technology, applied in the field of protein vaccines, to achieve the effect of reducing use, good effect and mature preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Industrial preparation of grass carp bacterial septicemia and red skin disease dual propolis inactivated vaccine
[0019] 1. Primary seed preparation
[0020] Take out the strains (Aeromonas hydrophila strain / Pseudomonas aeruginosa strain) stored in the refrigerator, inoculate nutrient agar, incubate at 28°C for 24 hours, inoculate nutrient broth 300ml / bottle, 10 bottles, shaker at 28°C , and cultivated at 200 rpm for 18 hours to obtain 3L of primary seed solution.
[0021] The strains selected in this example are: Aeromonas hydrophila strain GA201, isolated from grass carp; Pseudomonas aeruginosa JP802, isolated from grass carp; the current strains are all preserved in the Aquatic Vaccine Project of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences Technology Center.
[0022] 2. Secondary seed preparation
[0023] Prepare 20L of Aeromonas hydrophila / Pseudomonas aeruginosa fermentation medium according to the fermentation medium...
Embodiment 2
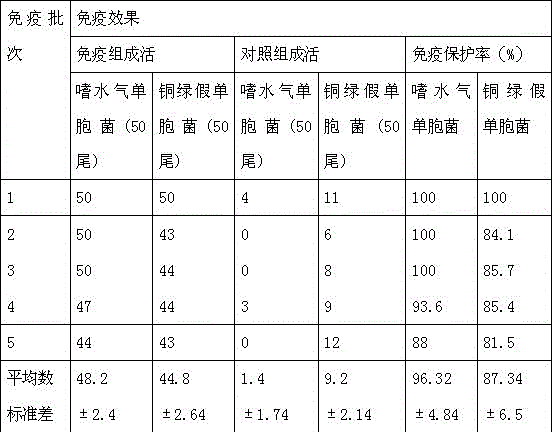
[0044] Example 2 The immune effect of grass carp bacterial sepsis, red skin disease dual propolis inactivated vaccine
[0045] A total of 5 batches of grass carp bacterial sepsis and red skin disease dual propolis inactivated vaccines were produced for immune experiments. For each batch of immunization experiments, healthy grass carp were randomly divided into 2 groups, 100 fish in each group. The first group was the immunization group, and each fish was intraperitoneally injected with the grass carp bacterial septicemia and red skin disease double propolis inactivated vaccine prepared in Example 1. 0.1ml; the second group is the control group. The water temperature is 25-28°C. After 21 days of immunization, inject 0.2ml of the virulent strain of Aeromonas hydrophila (containing about 1.8×107cfu of viable bacteria) / 0.2ml of the virulent strain of Pseudomonas aeruginosa ( Aeromonas hydrophila GA201 / Pseudomonas aeruginosa JP802 (the strains are stored in the Aquatic Vaccine En...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com