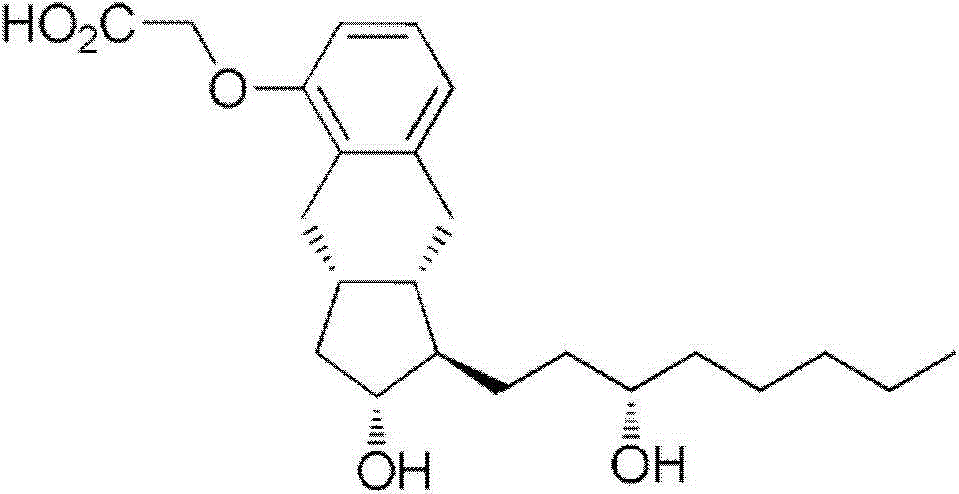
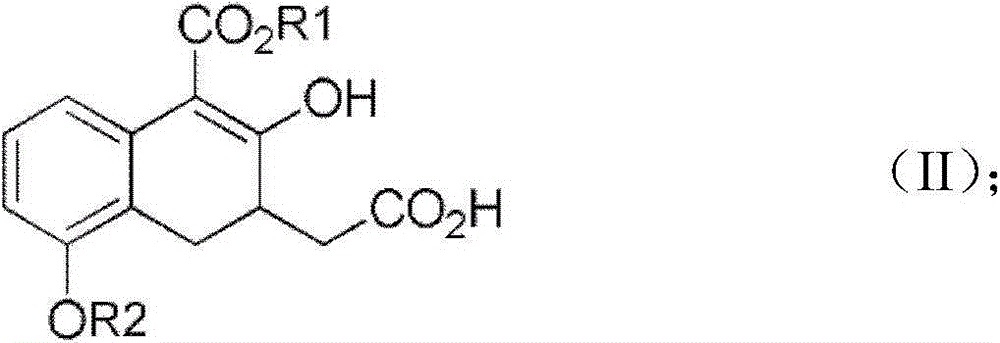Synthetic method for treprostinil diethanol amine and novel intermediate
A technology of treprostinil and synthesis method, applied in the field of synthesis of treprostinil diethanolamine, can solve problems such as time-consuming, complex synthesis method and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] [Example 1] - Preparation of Compound 1 - Methoxycarbonyl Reaction (i)
[0071]
[0072] Dissolve 500 grams of 5-methoxy-2-tetralone (5-methoxy-2-tetralone) in 3.75 liters of dimethylcarbonate (dimethylcarbonate), add 633 ml of 30% methanol at 15°C Sodium (CH 3 ONa) methanol solution, then heated to 70 ° C for 1 hour, after the reaction was cooled to room temperature, 1.2 liters of 3N hydrochloric acid aqueous solution was added to terminate the reaction, the organic layer was separated, and the aqueous layer was extracted with 1 liter of ethyl acetate. The combined organic layers were concentrated under reduced pressure. The obtained crude product was extracted with 3.29 liters of n-hexane, filtered, concentrated and dried to obtain 531 grams of compound 1 as a yellow solid.
Embodiment 2
[0073] [Example 2] - Preparation of Compound 2 - Alkylation Reaction (ii)
[0074]
[0075] Dissolve 143 ml of diisopropylamine (diisopropylamine) in 1 liter of tetrahydrofuran (THF), add dropwise 272 ml of 1.6M n-butyllithium (n-butyllithium) in n-hexane at -60°C, and React at 60°C for 15 minutes, then dropwise add 92 g of compound 1 dissolved in 600 ml of tetrahydrofuran (THF), and react at -60°C for 1 hour. Then, add 68 grams of lithium bromoacetate (lithium bromoacetate, ) and 29 grams of tetrabutylammonium iodide (tetrabutylammoniumiodide, TBAI), and reacted at room temperature for 22 hours. Then, 1.5 liters of 2N hydrochloric acid aqueous solution was added at 5°C to terminate the reaction. After the organic layer was separated, the organic layer was washed twice with 1.5 liters of 2N hydrochloric acid aqueous solution, and concentrated under reduced pressure to obtain 110 grams of off-white solid compound 2. Among them, the compound 2 is a preferred embodiment of ...
Embodiment 3
[0076] [Example 3] - Preparation of Compound 3 - Demethoxycarbonyl Reaction (1-1)
[0077]
[0078] Dissolve 340 g of compound 2 and 54 g of lithium chloride (LiCl) in dimethylacetamide (DMAc) / water (1.7 liters / 68 ml), heat to 100 ° C for 2 hours and then cool to room temperature . Add 1.5 liters of saturated brine and 1 liter of ethyl acetate to partition, wash the organic layer with 4.5 liters of saturated brine, and concentrate under reduced pressure to obtain 225 g of compound 3 as an orange solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com