Nucleic acid label and kit for auxiliary diagnosis of Kawasaki disease
A nucleic acid labeling, auxiliary diagnosis technology, applied in subjects of intervention, biomarkers, calcineurin 3 catalytic subunit gamma isoform protein gene, protein, kit for auxiliary diagnosis of Kawasaki disease, detection of KD biological Markers, drugs for the treatment of Kawasaki disease, 25% of KD children treated can develop permanent coronary artery damage, or its accelerator field, to predict whether there is concurrent coronary artery damage, stable results, and great clinical application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: 6 children with KD in the acute stage and 6 healthy children who visited the Children's Hospital Affiliated to Soochow University were selected as controls. Fasting blood was drawn in the morning, plasma was separated, and total RNA in the plasma was extracted, and miRNA chip hybridization technology was used to detect Kawasaki disease Differentially expressed miRNAs in the plasma of the control group and the normal control group. Eight children with acute KD and 8 healthy children were selected, and real-time quantitative PCR (real-time quantitative PCR, RT-qPCR) technology was used to verify the chip results. Three databases, TargetScan, PITA and microRNAorg, were used to predict target genes of differential miRNAs, and DAVID bioinformatics analysis software was used to perform GO and KEGG analysis on common target genes to find target genes related to KD vascular inflammation.
[0039] 1. The instrument involved in the embodiment
[0040] 1.1 Main instrum...
Embodiment 2
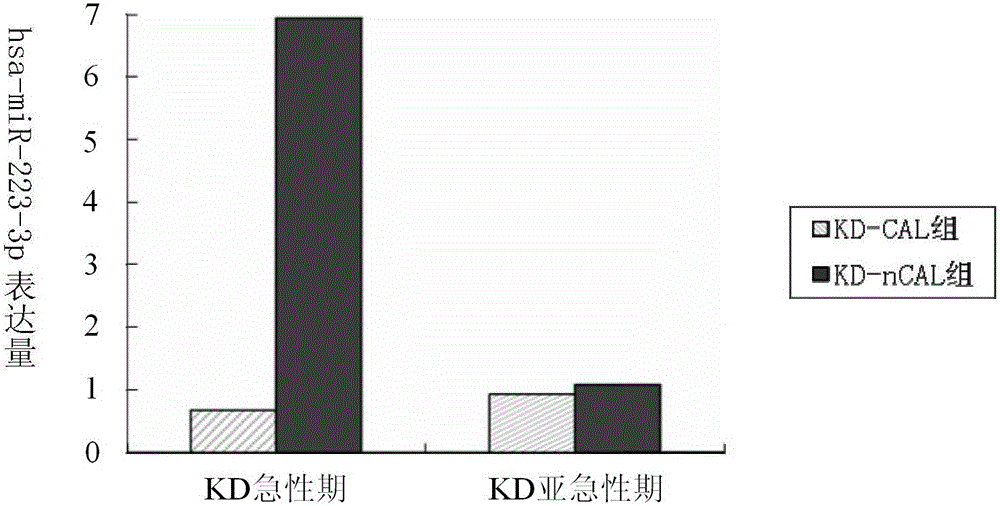
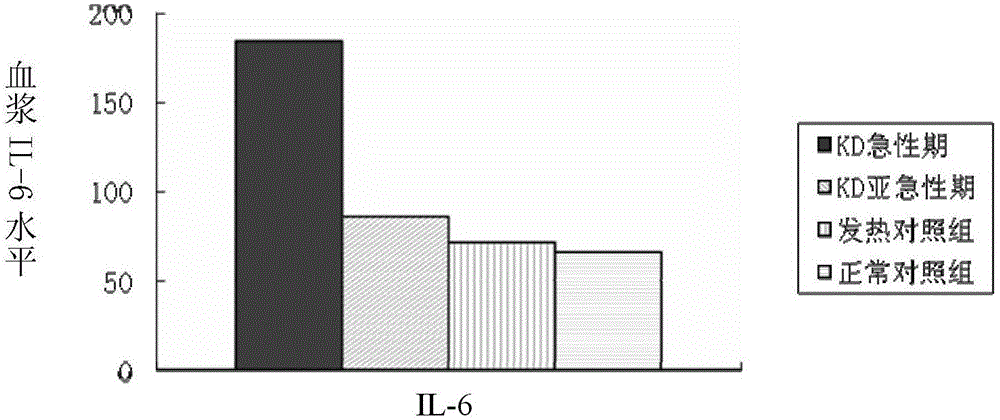
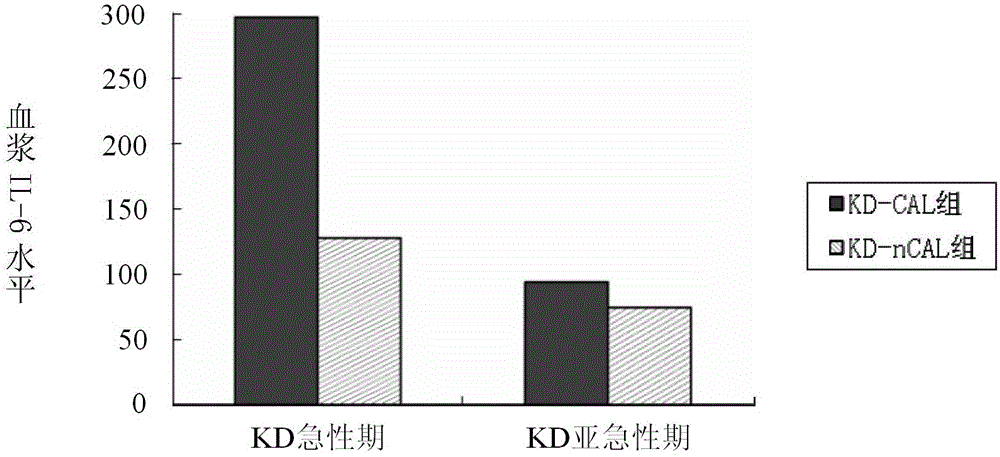
[0141] Example 2, taking hsa-miR-223-3p as the research object, to explore the expression of hsa-miR-223-3p in plasma before and after KD gamma globulin treatment and its correlation with the inflammatory cytokine IL-6. To explore the clinical significance of hsa-miR-223-3p detection in KD. Thirty children with KD were selected, including 10 patients with coronary artery injury and 20 patients with non-coronary artery injury. There were 12 cases of fever and normal control group. RT-qPCR was used to detect the expression of hsa-miR-223-3p in the acute phase and subacute phase of KD, and the level of IL-6 in plasma was detected by enzyme-linked immunosorbent assay (ELISA). Differences between groups were analyzed, and the correlation between hsa-miR-223-3p and IL-6 was analyzed.
[0142] 1. Instruments involved in the embodiment
[0143]
[0144]
[0145] Analysis software: AscentsoftwareforMultiskan
[0146] 2. Main reagents involved in the examples
[0147] RT-qPCR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com